Personalized Gene Therapy Treatments For Cancer Market Size and Forecast – 2026 – 2033
The Global Personalized Gene Therapy Treatments For Cancer Market size is estimated to be valued at USD 9.8 billion in 2026 and is expected to reach USD 27.5 billion by 2033, exhibiting a compound annual growth rate (CAGR) of 15.4% from 2026 to 2033.
Global Personalized Gene Therapy Treatments For Cancer Market Overview
Personalized gene therapy treatments for cancer are advanced therapeutic solutions tailored to an individual’s genetic tumor profile. These products use genetic engineering technologies such as viral vectors, CRISPR-based systems, or synthetic DNA/RNA constructs to modify or correct genetic abnormalities that drive cancer progression. They may involve inserting therapeutic genes, silencing oncogenes, or enhancing immune response via engineered T-cells. Personalized gene therapies are typically developed after detailed genomic profiling of a patient’s tumor to identify actionable mutations. They are administered as targeted infusions or injections in specialized clinical settings, often as part of precision oncology programs.
Key Takeaways
Hematologic cancer leads the cancer type segment, capturing the highest market share (52%), driven by more mature gene therapy modalities and reimbursement acceptance.
Solid tumors are rapidly growing, particularly in the Asia Pacific, presenting compelling market growth opportunities.
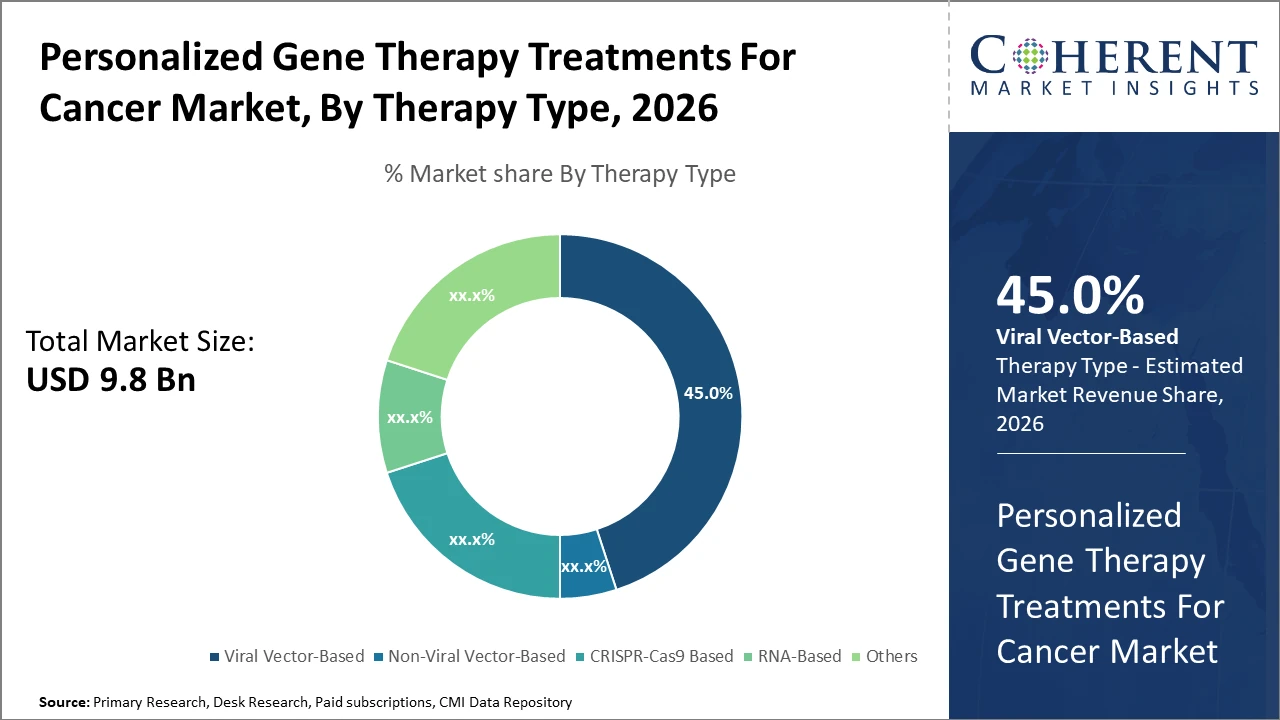
Viral vector-based therapies dominate therapy type segmentation with a 45% market share, reflecting their clinical efficacy and regulatory familiarity, though CRISPR-Cas9 therapy is the fastest growing due to precision and versatility.
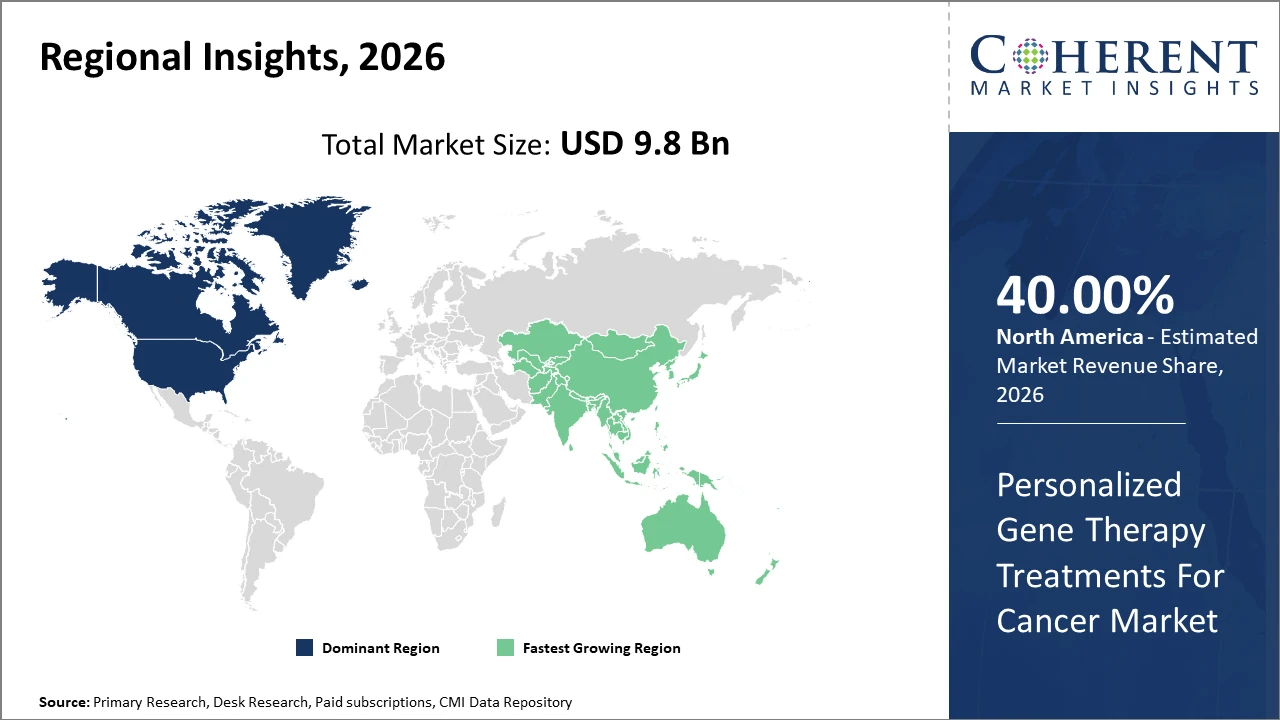
North America maintains market dominance with advanced manufacturing infrastructure and supportive regulatory frameworks, accounting for over 40% of the market share.
The Asia Pacific region shows the fastest CAGR, fueled by burgeoning oncology prevalence and government health initiatives in countries like China and India.
Personalized Gene Therapy Treatments For Cancer Market Segmentation Analysis
To learn more about this report, Download Free Sample
Personalized Gene Therapy Treatments For Cancer Market Insights, By Therapy Type
Viral Vector-Based therapies dominate the market share with 45%. This dominance arises from well-established clinical efficacy, familiarity among regulatory bodies, and existing manufacturing capabilities. Viral vector therapies are preferred for their reliable gene delivery mechanisms in hematologic cancers, enabling high gene expression and persistent therapeutic effects. The fastest-growing subsegment is CRISPR-Cas9-based therapy. Its precision gene-editing capacity offers immense promise in targeting cancer-specific mutations with minimal off-target effects.
Clinical trials in 2025 demonstrated CRISPR-based treatments leading to complete remission in certain refractory cancers, highlighting its disruptive potential. Non-Viral Vector-Based therapies are progressing steadily due to lower immunogenicity, but face scalability issues. RNA-based therapies provide flexible modulation of gene expression and are increasingly explored for solid tumors.
Personalized Gene Therapy Treatments For Cancer Market Insights, By Cancer Type
Hematologic Cancer dominates at 52% market share due to early clinical successes and regulatory approvals in treatments like CAR-T cell therapies. These therapies show high remission rates in leukemias and lymphomas and have set the benchmark for personalized gene therapy efficacy. The mature reimbursement framework supports this dominance. Solid Tumors represent the fastest-growing subsegment, driven by breakthroughs in tumor microenvironment targeting and enhanced delivery systems. Advances in intratumoral delivery mechanisms in 2026 have improved gene therapy accessibility for difficult-to-treat cancers such as pancreatic and lung cancers. Rare Cancers and Others maintain niche growth supported by orphan drug designations and innovative trial designs, which progressively expand market scope.
Personalized Gene Therapy Treatments For Cancer Market Insights, By End-User
Hospitals & Specialty Clinics commanding a 60% market share. This reflects a direct patient care focus and integration with clinical trial frameworks, enabling quicker adoption and commercial scaling of personalized gene therapies. The fastest-growing segment is the CDMOs subsegment, which benefits from outsourcing trends due to the technical complexity and cost of manufacturing gene therapies. Increased demand for specialized manufacturing and scaling facilities was evident in 2025 when production volume grew by over 28%. Research Institutes remain instrumental in driving early-stage innovation and validation trials, serving as a crucial feeder pipeline for industrial-scale development.
Personalized Gene Therapy Treatments For Cancer Market Trends
The Personalized Gene Therapy Treatments For Cancer market’s evolution in recent years highlights several distinct trends.
First, the rise of allogeneic gene therapies reduces manufacturing complexities and offers scalable treatment models, as seen by the launch of the first allogeneic CAR-T therapies in 2025.
This trend aligns with growing regulatory approvals favoring modular and off-the-shelf therapies.
Second, the integration of AI and big data analytics has revolutionized patient stratification and real-time genomic profiling.
Notably, oncology centers reported a 20% increase in successful personalized treatment regimens from 2024 to 2026 due to enhanced AI-supported diagnostics.
Personalized Gene Therapy Treatments For Cancer Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Personalized Gene Therapy Treatments For Cancer Market Analysis and Trends
In North America, dominance in the Personalized Gene Therapy Treatments For Cancer market is fueled by substantial R&D investments, robust regulatory frameworks, and extensive biopharmaceutical manufacturing ecosystems. The region holds over 40% market share sustained by leadership from key U.S. entities like Novartis and Gilead Sciences. Favorable reimbursement policies and ongoing clinical trial activity continue to strengthen its infrastructure and market position.
Asia Pacific Personalized Gene Therapy Treatments For Cancer Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth with an estimated CAGR exceeding 18% through 2033. Factors driving this include rising cancer prevalence, expanding healthcare infrastructure, and increasing government-funded oncology programs, particularly in China and India. The presence of emerging biotech companies and the uptake of personalized gene therapies in metropolitan hospitals enhance market growth potential.
Personalized Gene Therapy Treatments For Cancer Market Outlook for Key Countries
USA Personalized Gene Therapy Treatments For Cancer Market Analysis and Trends
The USA market leads due to its pioneering technological innovations and highest clinical trial volume globally. In 2025, the U.S. accounted for over 50% of worldwide personalized gene therapy clinical trials for cancer, emphasizing its central position. Key companies such as Novartis and Kite Pharma have propelled advancement through FDA approvals of multiple therapies, driving significant revenue growth. Additionally, government initiatives like the Cancer Moonshot bolster the ecosystem, sustaining high market growth and supporting extensive diagnostics and manufacturing capabilities.
China Personalized Gene Therapy Treatments For Cancer Market Analysis and Trends
China's Personalized Gene Therapy Treatments For Cancer market is rapidly expanding, supported by a favorable regulatory environment and enhanced government funding for biopharmaceutical innovation. The Chinese government has introduced targeted incentives for gene therapy R&D since 2024, accelerating clinical trial pipelines and launch approvals. Leading domestic firms are partnering with multinational corporations to develop locally relevant treatments, expanding market scope and demand. The growing oncology patient population further facilitates robust market growth supported by infrastructure developments across tier-1 and tier-2 cities.
Analyst Opinion
Increasing Manufacturing Capacity and Innovation Drives Market Revenue Growth: The surge in personalized gene therapy manufacturing capacities, particularly in North America and Europe, has significantly bolstered market revenue. For instance, major biopharmaceutical manufacturing plants expanded capacity by over 30% in 2024 alone, facilitating faster gene vector production and enhancing supply-side dynamics. This capacity scale-up directly supports higher market share and business growth while mitigating previous supply constraints in 2025.
Expanding Clinical Applications Accelerate Market Demand: The use of personalized gene therapies across various cancer types—such as hematologic malignancies and solid tumors—is broadening. Data from 2025 reveals over 75% growth in clinical trial initiations targeting pediatric and adult oncology segments, indicating a diverse and rapidly growing demand landscape. This diversification catalyzes market growth strategies and deepens market insights into therapeutic applicability.
Rising Adoption of Combination Therapies Enhances Market Scope: Recent 2026 clinical trials show that combination approaches, integrating gene therapy with immune checkpoint inhibitors or chemotherapy, improve efficacy, leading to increased physician adoption and payer acceptance. This multiplier effect on market growth directly impacts the market size and market forecast positively, notably in advanced-stage cancer treatments.
Pricing Flexibility and Reimbursement Landscape Support Market Expansion: Despite the high cost perception, evolving reimbursement schemes and value-based pricing models throughout 2024-2026 have improved patient access. For example, select regions in Europe and North America implemented innovative reimbursement policies that increased therapy adoption rates by 20%-25% year-over-year, fueling overall market dynamics and business growth.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 9.8 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 15.4% | 2033 Value Projection: | USD 27.5 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Takeda Pharmaceutical, Celyad Oncology, Audentes Therapeutics, PTC Therapeutics, Beam Therapeutics, Moderna Therapeutics, MeiraGTx, Regenxbio, Homology Medicines, TCR2 Therapeutics | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Personalized Gene Therapy Treatments For Cancer Market Growth Factors
The expanding biotechnological innovation pipeline fosters continuous therapeutic advancements, particularly in areas involving CRISPR-based precision editing techniques. The unprecedented specificity and efficacy improvements realized in 2025 clinical results have markedly bolstered market growth. The rising prevalence of cancer globally propels demand, as WHO data indicated that cancer incidence surpassed 20 million new cases in 2024, underscoring an urgent need for personalized treatment modalities. Additionally, government initiatives and increased R&D funding, like the U.S. Cancer Moonshot program’s multibillion-dollar allocation in 2025, have stimulated intensified development efforts, further catalyzing market dynamics. Enhanced real-world evidence adoption facilitates payer confidence and regulatory approvals, smoothing market entry paths and improving forecasted market size.
Personalized Gene Therapy Treatments For Cancer Market Development
In October 2024, India officially launched NexCAR19, the country’s first indigenously developed and affordable CAR-T cell therapy, with the inauguration carried out by the President of India. Developed through a collaboration between IIT Bombay, Tata Memorial Centre, and ImmunoACT, NexCAR19 significantly reduced treatment costs compared to imported CAR-T therapies, making personalized cellular immunotherapy accessible to a much broader patient population.
In 2023, GenoCure Pharmaceuticals introduced a new gene-based therapeutic strategy targeting solid tumors, addressing long-standing delivery and efficacy challenges associated with gene therapies beyond hematological cancers. In parallel, ThermaGene Therapeutics launched advanced gene delivery systems designed to improve transfection efficiency, tissue specificity, and safety profiles, supporting the broader transition of gene therapies from experimental concepts to scalable, clinically viable treatment modalities.
Key Players
Leading Companies of the Market
Takeda Pharmaceutical
Celyad Oncology
Audentes Therapeutics
Beam Therapeutics
Moderna Therapeutics
MeiraGTx
Regenxbio
Homology Medicines
TCR2 Therapeutics
Several market players have adopted targeted collaboration and licensing strategies to accelerate pipeline development and market penetration. For example, in 2025, Novartis partnered with Sangamo Therapeutics to co-develop gene editing therapies, which significantly improved their pipeline throughput and positioned them for a 12% market share increase in North America. Similarly, CRISPR Therapeutics’ strategic alliance with Vertex Pharmaceuticals yielded promising clinical trial outcomes in 2024, enabling expanded commercial readiness.
Personalized Gene Therapy Treatments For Cancer Market Future Outlook
The future of personalized gene therapy for cancer is poised for robust growth as technological advancements continue to reduce barriers to clinical translation. Continued refinement of delivery systems — including non-viral vectors and targeted nanoparticles — will enhance safety and enable broader application beyond hematologic malignancies into solid tumors. Integration of next-generation sequencing with artificial intelligence will accelerate the identification of actionable genetic changes, enabling truly individualized treatment designs.
Regulatory frameworks are also evolving to better accommodate gene therapies, with accelerated approval pathways and adaptive trial designs that prioritize patient outcomes. As manufacturing processes scale up and costs decrease, access to these highly individualized treatments is expected to expand globally. Collaboration between academic institutions, biotech startups, and large pharmaceutical companies will further diversify the therapeutic offerings, positioning personalized gene therapy as a cornerstone of future oncologic care.
Personalized Gene Therapy Treatments For Cancer Market Historical Analysis
The Personalized Gene Therapy Treatments For Cancer Market emerged from decades of foundational research in molecular biology, immunology, and genetic engineering. Early cancer treatments were dominated by surgery, radiation, and non-specific chemotherapies, which often produced significant side effects due to lack of target specificity. The discovery of oncogenes and tumor suppressor genes in the late 20th century laid the groundwork for targeted therapies. However, it was not until the development of viral vector technologies and advances in gene editing — such as CRISPR/Cas9 — that personalized gene therapy became a realistic therapeutic modality.
Initial clinical trials in the early 2000s focused on rare genetic diseases, gradually expanding into oncology as proof-of-concept studies demonstrated the feasibility of modifying patient T-cells to recognize and kill tumor cells. The approval of the first CAR-T cell therapies in the 2010s marked a watershed moment, establishing gene therapy as a viable category of cancer treatment. With improvements in vector safety, delivery mechanisms, and genomic profiling, the market transitioned from academic curiosity to commercial pipeline development, supported by regulatory pathways that recognize the urgency of precision cancer medicine.
Sources
Primary Research Interviews:
Oncologists
Geneticists
gene therapy researchers
biotech executives
clinical trial investigators
Databases:
ClinicalTrials.gov
NIH Cancer Genome Atlas
FDA Gene Therapy Database
GlobalData Pharma
Magazines:
Nature Biotechnology
BioPharma Dive
Genetic Engineering & Biotechnology News
Drug Discovery Today
Pharmaceutical Technology
Journals:
Nature Medicine
Journal of Clinical Oncology
Molecular Therapy
Cancer Research
The Lancet Oncology
Newspapers:
Financial Times (Biotech)
Reuters Pharma
The New York Times (Science)
Bloomberg Healthcare
The Guardian (Science)
Associations:
American Society of Clinical Oncology
Biotechnology Innovation Organization
International Society for Cell & Gene Therapy
FDA
EMA
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients