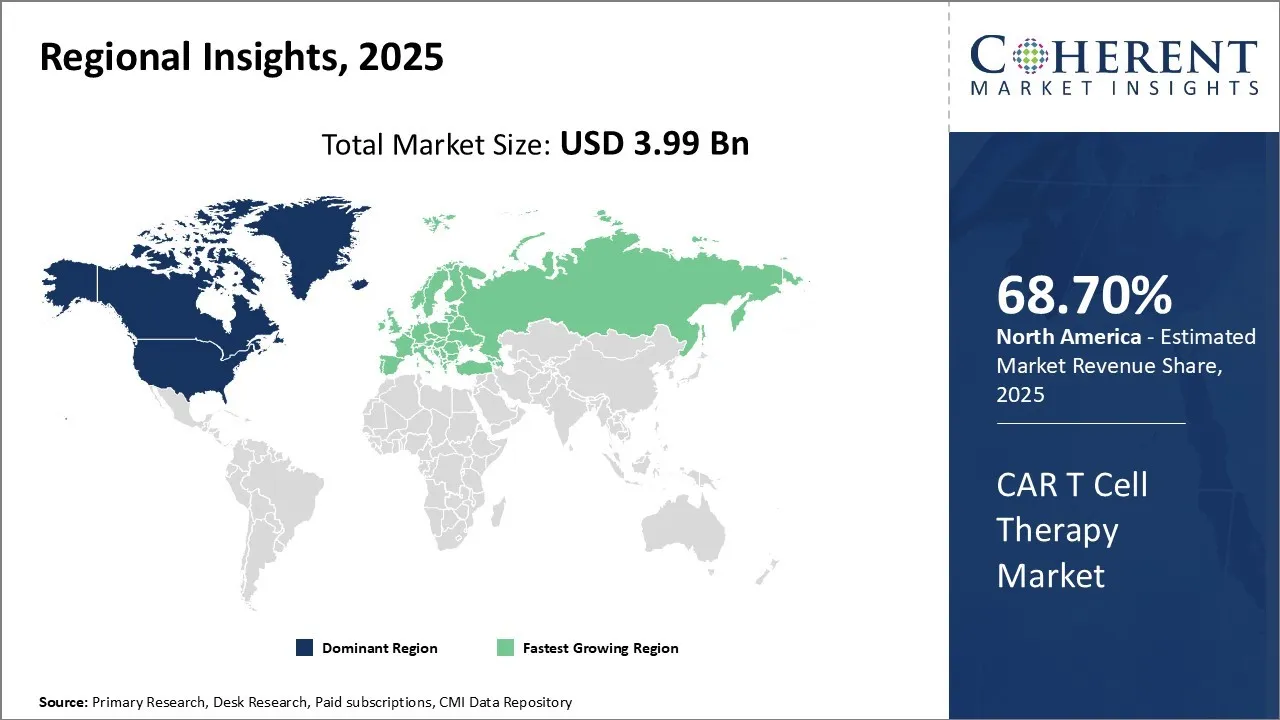
Car T Cell Therapy Market is estimated to be valued at USD 3.99 Bn in 2025 and is expected to reach USD 15.06 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 20.9% from 2025 to 2032.
The international CAR T cell therapy market is gaining strong growth with the expanding base of hematologic cancers, growing R&D investment in immunotherapy, and the widening pipeline of CAR T products. New advances in cell engineering, manufacturing, and regulatory support are facilitating broader clinical use of the therapy, particularly for refractory conditions or Car T cell therapy of relapse cases. Moreover, second-generation CAR T therapies such as allogeneic platforms will become scalable and accessible in the future.
|
Current Events |
Description and its impact |
|
Legend Biotech and Janssen Expand Use of CARVYKTI in 2025 |
|
|
Autolus Therapeutics Gets FDA Approval for Aucatzyl in 2024 |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The market of CAR T cell therapy is characterized by very high treatment costs, mainly due to the advanced, highly tailored manufacturing process, sophisticated logistics, and strict regulatory adherence involved in producing the therapies. Recent prices for approved CAR T cell treatments, such as Kymriah (Novartis), Yescarta (Gilead/Kite Pharma), and Breyanzi (Bristol Myers Squibb), range from $373,000 to $475,000 per cycle of treatment in the United States, excluding inpatient hospital stays and supportive care.
The key cost driver is the autologous nature of current CAR T therapies, which involves taking the patient's own T cells, altering them ex vivo, and readministering them. These processes are time, labor, and facility-intensive, all contributing factors to the cost drivers. The lack of economies of scale, due to the one-off production process, also keeps prices high.
Reimbursement support from public and private payers in the U.S. and EU is increasing, especially in the case of relapsed or refractory blood cancers. It still remains limited in low- and middle-income countries due to budget limitations. Outcome-based reimbursement and value-based models are in discussions to aid high expenditures by linking payment with clinical effectiveness.
The direction of CAR T therapy prices can be altered with off-the-shelf (allogeneic) CAR T therapies available, which promise mass production and lower cost-per-dose. As more players enter the market and technology advances, the market will gradually reduce prices and extend access globally.
Artificial Intelligence (AI) is increasingly being employed to transform the CAR T cell therapy industry, addressing many of the key challenges across the therapy life cycle—ranging from discovery and manufacturing to patient identification and monitoring post-treatment. With the CAR T therapies being so personalized and complicated, AI offers the possibility of improving precision, efficiency, and scalability at every stage.
During research and development, AI algorithms are used to analyze large genomic and proteomic data to decide optimal CAR constructs, predict optimal antigen targets, and design improved T cell receptors. This accelerates drug development timelines and enhances cell therapy candidates before expensive clinical trials.
In manufacturing, AI-driven automation is helping optimize cell process workflows, reducing errors, maintaining product quality consistency, and maximizing throughputs in GMP facilities. AI solutions can detect and prevent production failure by tracking such factors as cell viability, transduction efficiency, and expansion rates.
In the clinic, AI is used for predictive analysis to identify CAR T Cell Therapy patient eligibility by determining which patients are most likely to benefit from the treatment and which are at higher risk of side effects such as cytokine release syndrome (CRS). This enables individualized treatment planning and risk reduction.
Post-treatment, AI enables real-time monitoring through the incorporation of electronic health records and wearable technology to track response and relapse risk. Overall, AI is enhancing the effectiveness, safety, and availability of CAR T therapies, and will play a key role in enabling future-generation cost-effective and available cell therapies.
Large cancer centers and hospitals report that CAR T cell therapy is a great advancement in relapsed or refractory cancer patients. Healthcare workers emphasize its life-saving potential, especially in hematological cancers when standard treatments have failed. They do, however, point out practical challenges like complex cell manipulation, need for trained staff, and extended patient monitoring due to risks like cytokine release syndrome (CRS). Hospitals confirm additional growth in spite of these obstacles due to solid clinical outcomes.
Educational institutions see CAR T therapy as a top translational research priority. Researchers and clinician-scientists appreciate the potential to engineer customized CAR constructs and open first-in-human trials. They generally mention issues with funding deficits, regulatory challenges, and limited scalability of academic production. However, they remain optimistic due to rising biotech industry collaborations and government support.
Niche, specialty clinics adopt CAR T therapy for the ability to attract high-spending patients and differentiate their business in competitive healthcare markets. Feedback anticipates next-generation, off-the-shelf (allogeneic) CAR T products with faster turnaround and reduced infrastructure needs. Reimbursement delays and training needs are still a concern.
Market participants see favorable commercial opportunities but see limitations such as sophistication of manufacture, regulatory setting, and cost pressure. They are making strategic investments in automation, AI, and allogeneic platforms to improve access, decrease costs, and expand indications, reflecting clinician and payer feedback to drive more widespread adoption.
Increasing prevalence of cancer is expected to propel growth of the global CAR T cell therapy market during the forecast period. Furthermore, key companies focusing on product approval is expected to propel growth of the global CAR T cell therapy market during the forecast period. On November 8, 2024, Autolus Therapeutics received U.S. FDA approval for its CAR T cell therapy Acetyl (obecabtagene autoleucel) to treat adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL). Aucatzyl is a CD19-directed CAR T therapy and the first approved treatment developed by Autolus.
The major factor that hinders growth of the global CAR T cell therapy market include the side effects of CAR T cell therapy. For instance, in March, 2022, American Cancer Society, a nationwide voluntary health organization, published a data according to which there are many possible side effects of CAR T cell therapy which includes: high fever and chill, trouble breathing, severe nausea, vomiting, and/or diarrhea, feeling dizzy or lightheaded, headaches, fast heartbeat, feeling very tired, muscle and/or joint pain etc.
CD19 leads the CAR T Cell Therapy market with an 86.5% share because it is a well-established, highly specific antigen expressed on most B-cell malignancies, including acute lymphoblastic leukemia and certain lymphomas. Targeting CD19 allows effective elimination of malignant B cells while sparing most other healthy cells.
Extensive clinical trials and FDA approvals for CD19-directed therapies have built strong confidence among clinicians and patients. Additionally, CD19 CAR T therapies have demonstrated impressive remission rates and Car T Cell Therapy with safety profile. This combination of proven efficacy and regulatory support sustains its market dominance.
To learn more about this report, Download Free Sample
North America dominates the CAR T Cell Therapy Market with a 68.70% share due to its advanced healthcare infrastructure, strong biopharmaceutical industry, and early adoption of innovative therapies. The U.S. leads in research funding, clinical trials, and regulatory approvals, accelerating market growth. Additionally, higher patient awareness and better reimbursement policies support widespread therapy adoption. These factors collectively establish North America as the key regional leader.
The U.S. dominates most of the world's CAR T cell therapy market with a regulatory approval head start, high R&D expenditures, and access to giant biopharmaceuticals like Novartis, Gilead, and Bristol Myers Squibb. A smooth FDA approval process, advanced healthcare infrastructure, and supportive reimbursement policies have encouraged the high uptake of CAR T therapies in the treatment of blood cancers.
China is becoming increasingly a market leader for CAR T cell therapy with massive government investments, a huge patient population, and an eased regulatory environment for new therapies. More than 400 trials are ongoing related to CAR T, and domestic biotechs like JW Therapeutics and Legend Biotech are leading both autologous and allogeneic therapies.
Germany is Europe's biggest health market and plays a central role in the use of CAR T therapy in the continent. It is blessed with early access to EMA-approved therapies, adequate reimbursement mechanisms through statutory health insurance, and interlinkage between hospitals, universities, and biopharmaceutical players that enables clinical research and patient access to flourish.
Japan is slowly growing in the CAR T market, and approvals of CAR T treatments are being given by the Ministry of Health, Labour and Welfare (MHLW) under fast-track designations. Companies like Daiichi Sankyo and Takeda are developing capabilities locally, supported by the aging population in Japan and increased cancer incidence.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 3.99 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 20.9% | 2032 Value Projection: | USD 15.06 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Bristol-Myers Squibb Company, Johnson & Johnson Services, Inc., Novartis AG, CARsgenTherapeutics Co., Ltd, Aurora Biopharma, Legend Biotech, Gilead Sciences, Inc., Pfizer Inc., bluebird bio, Inc., Sorrento Therapeutics, Inc., Mustang Bio, Fate Therapeutics |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients