Viral Vector and Plasmid DNA Manufacturing Market-Market Insights
Viral vectors are used as carriers to transfer the genetic material into other living organisms. The viral vector production can be done in two types of culture system such as suspension cell cultures and adherent-cell systems. For large-scale manufacturing of viral vectors, the conventional lab processes of adherent-cell systems such as multilayer flasks, hollow-fiber technologies, T-flasks, and roller bottles experience challenges in scaling up such as larger space requirement and increased manual workload.
Plasmid DNA is extrachromosomal DNA found in bacteria, which is generally circular in shape. The genetic engineers use plasmid DNA to create recombinant DNA, in order to transfer genetic material in other organisms. Plasmid DNAs are limited by some biological barriers such as endosomal attack, renal clearance, and degradation by serum endonucleases. Moreover, for direct gene transfer into humans, good manufacturing practice (GMP)-grade plasmid DNA is mandatory. Therefore, around 70% of gene therapy clinical trials, to date, have involved viral vector systems.
The global viral vector and plasmid DNA manufacturing market is expected to be valued at US$ 427.2 million in 2019, and is expected to exhibit a CAGR of 22.8% over the forecast period (2019-2027).
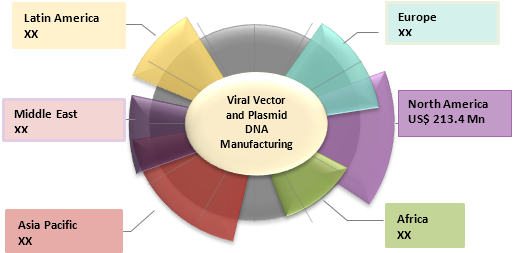
Figure 1. Global Viral Vector and Plasmid DNA Manufacturing Market Value (US$ Mn), by Region, 2019
To learn more about this report, Download Free Sample
Source: Coherent Market Insights Analysis (2020)
Increasing number of gene therapy patients is expected to propel the market growth over the forecast period
The increasing number of patients opting for gene therapy for treating diseases such as cancer, cystic fibrosis, heart disease, diabetes, hemophilia, and AIDS is expected to drive growth of the viral vector and plasmid DNA manufacturing market over the forecast period. For instance, Adeno-associated AAV2 vectors carrying therapeutic gene (RPE65) intra-retinal injection lead to improve vision of individuals suffering from Leber’s Congenital Amaurosis.
Moreover, several clinical trials are being conducted on viral vectors and plasmid DNA manufacturing that are focused on the potential of gene therapy. According to a data published by the Journal of Gene Medicine, in 2017, around 2,600 gene therapy clinical trials are ongoing, have been completed or approved in 36 countries. Moreover, countries, where the trials were conducted include the U.S., U.K., Australia, Canada, China, France, Germany, Japan Switzerland, the Netherlands, and others.
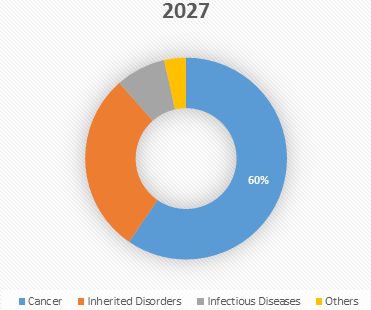
Figure 2. Global Viral Vector and Plasmid DNA Manufacturing Market Share (%), by Therapeutic Application, 2027
To learn more about this report, Download Free Sample
Source: Coherent Market Insights Analysis (2020)
Increasing technological advancements in the manufacturing of viral vector and plasmid DNA is expected to drive the market growth over the forecast period
The rising demand for viral vector and plasmid DNA for gene therapies has led major players in the market to launch new and technologically advanced programs and devices to scale up the production of viral vectors and plasmid DNA. For instance, in April 2018, GE Healthcare Life Sciences launched a prefabricated, modular bioprocessing facility KUBio BSL 2 for production of viral vector-based vaccines, oncolytic virus, and cell and gene therapies.
Moreover, government funding is increasing for development of new technologies and production facilities of viral vector. For instance, in 2018, Innovate UK, Department for Business, Energy & Industrial awarded the Industrial Strategy Challenge Fund of US$ 3.4 million capital to Cobra Biologics for infrastructure investment to expand manufacturing capabilities and commercialization of DNA and viral vectors products. Thus, rising research and development funding from government organization is expected to drive growth of the market over the forecast period.
However, the manufacturing processes of viral vectors is expensive and time consuming. Large scale production of recombinant viral vector is a complex process and is considered as a major challenge and large barrier for commercialization. Therefore, increasing difficulties in viral vector manufacturing capacity is expected to hinder growth of the global viral vectors and plasmid DNA manufacturing market.
Key Players
Major players operating in the global viral vector and plasmid DNA manufacturing market include Lonza Group AG, FinVector Vision Therapies, Cobra Biologics and Pharmaceutical Services, Sigma-Aldrich Co. LLC, VGXI, Inc., VIROVEK, SIRION Biotech GmbH, FUJIFILM Diosynth Biotechnologies U.S.A., Inc., Sanofi, Cell and Gene Therapy Catapult, Brammer Bio, and MassBiologics.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients