Interspinous Spacers Market Size and Forecast – 2025 – 2032
The Global Interspinous Spacers Market size is estimated to be valued at USD 480 million in 2025 and is expected to reach USD 875 million by 2032, exhibiting a compound annual growth rate (CAGR) of 8.4% from 2025 to 2032.
Global Interspinous Spacers Market Overview
Interspinous spacers are implantable medical devices designed to relieve lumbar spinal stenosis by gently separating vertebrae and reducing nerve compression. Typically made of biocompatible metals or polymers, they are inserted between spinous processes to maintain spinal canal space, improve mobility, and minimize pain without requiring extensive spinal fusion surgery.
Key Takeaways
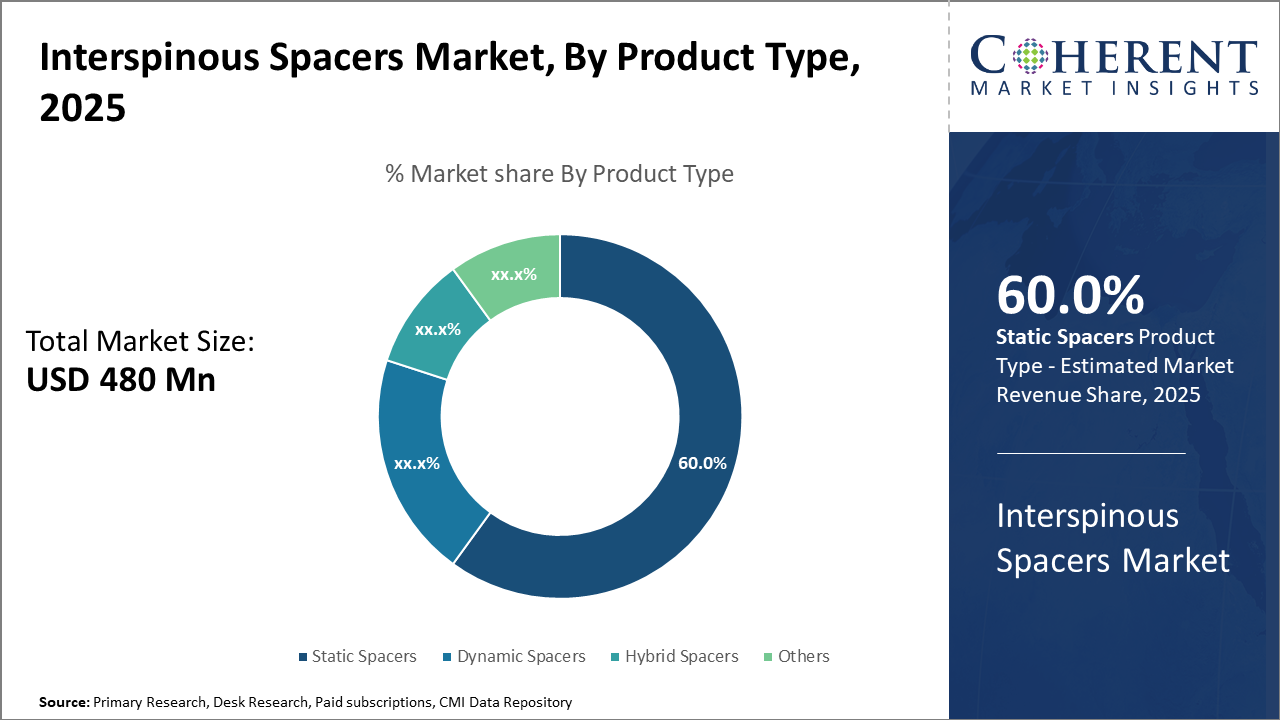
The Static Spacers segment dominates the product type category with 60% market share due to proven clinical efficacy and ease of implantation, driving revenue generation.
Hospitals serve as the largest end-user segment, attributed to their comprehensive surgical capabilities and growing spinal disorder surgical caseloads.
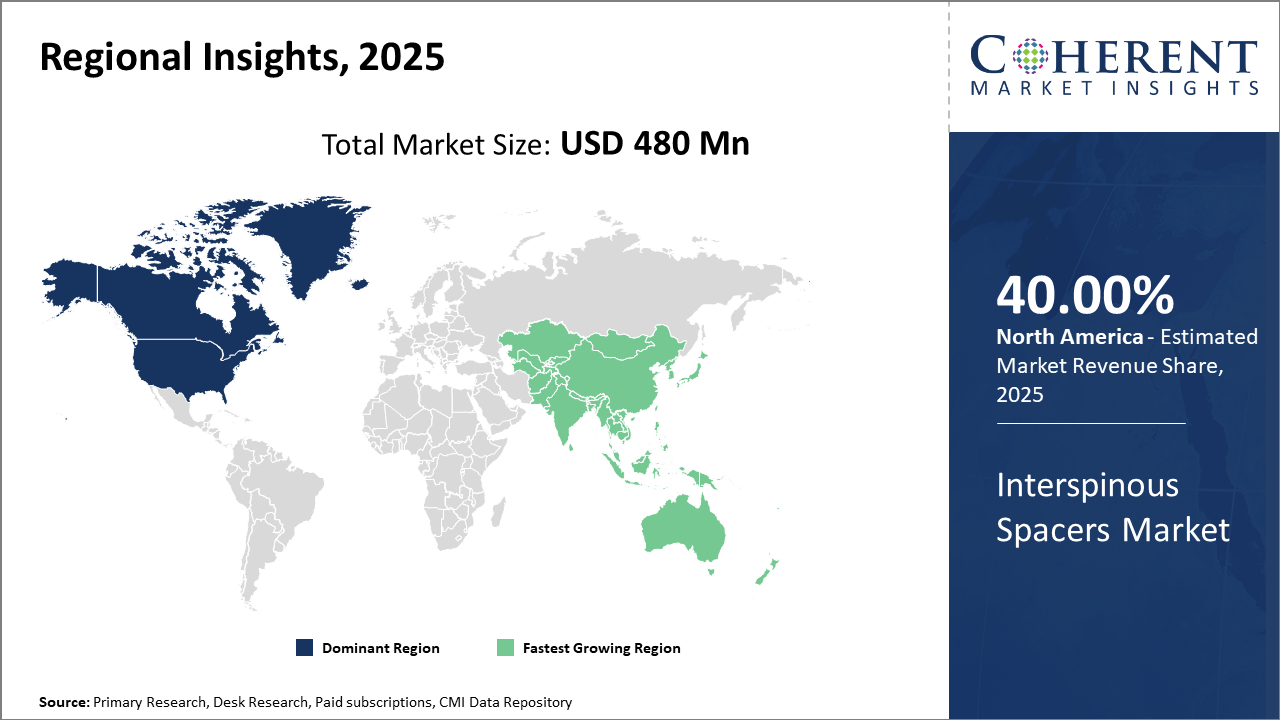
North America leads the regional market with approximately 40% industry share, underpinned by high healthcare expenditure and technological advancement in spinal care.
Asia Pacific stands out as the fastest-growing regional market with a CAGR exceeding 10%, stimulated by improving healthcare infrastructure and increasing patient awareness.
Europe maintains steady growth, supported by an established spinal implant manufacturing base and progressive government healthcare policies.
Interspinous Spacers Market Segmentation Analysis
To learn more about this report, Download Free Sample
Interspinous Spacers Market Insights, By Product Type
Static Spacers hold a commanding position due to their straightforward design, reliable biomechanical support, and extensive clinical validation, making them the preferred choice for most lumbar spinal stenosis treatments. Their structural stability reduces vertebral compression, minimizing patient postoperative complications, which fuels widespread hospital adoption. Meanwhile, Dynamic Spacers represent the fastest growing subsegment as they address limitations of static devices by allowing controlled spinal motion, thus preserving segment mobility and reducing stress on adjacent vertebrae. This subsegment’s growth is driven by increased physician interest in motion-preserving surgical options and favorable clinical trial results reported in 2024.
Interspinous Spacers Market Insights, By End-User Insights
Hospitals emerge as the dominant end-user segment, hosting the majority of lumbar spinal surgeries owing to their well-established surgical infrastructure, comprehensive postoperative care, and high procedure volumes. Their ability to support complex cases positions them as the epicenter of market revenue for Interspinous Spacers. Ambulatory Surgical Centers represent the fastest growing end-user segment, attributed to healthcare cost containment and increased patient preference for minimally invasive outpatient procedures. ASCs are expanding their capabilities to deliver spinal interventions with shorter recovery times, supported by favorable reimbursement for outpatient surgeries, thereby driving segment growth.
Interspinous Spacers Market Insights, By Application
Lumbar Spinal Stenosis commands a dominant market share, driven by its high prevalence among aging populations globally, and the well-documented effectiveness of Interspinous Spacers in alleviating neurogenic claudication symptoms. The wide clinical acceptance in this application supports sustained revenue generation and large-scale market penetration. Degenerative Disc Disease represents the rapidly growing application subsegment, propelled by advancements in spacer designs that accommodate the biomechanical challenges of degenerative spinal segments. Increasing diagnosis rates and therapeutic innovation underscore its escalating market presence.
Interspinous Spacers Market Trends
Market trends emphasize a transition toward advanced biocompatible materials and customized implant solutions, driven by rising clinician demand for improved spinal stabilization and patient outcomes.
In 2024, clinical studies demonstrated reduced surgical revision rates with bioresorbable spacers compared to traditional devices, fostering accelerated market acceptance.
Additionally, digital integration such as augmented reality-assisted spinal procedures is gaining momentum, improving precision and reducing operation times, particularly in developed markets.
These trends indicate a technologically driven market evolution.
Interspinous Spacers Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Interspinous Spacers Market Analysis and Trends
In North America, the dominance in the Interspinous Spacers market stems from extensive healthcare infrastructure, high medical device adoption rates, and supportive reimbursement frameworks. The region holds approximately 40% of the industry share, propelled by the US, where over 65,000 spinal stenosis surgeries incorporate Interspinous Spacers annually. Leading regional market players such as Medtronic and NuVasive have extensive operations here, driving innovation and business growth.
Asia Pacific Interspinous Spacers Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth, with a CAGR exceeding 10%, fuelled by expanding hospital networks and rising awareness about minimally invasive spinal treatments in countries like China and India. Government initiatives aimed at upgrading healthcare infrastructure, coupled with increased spine surgery volumes, support market expansion. Regional companies and multinational market players collaborate to capture this growth by tailoring solutions for local clinical needs.
Interspinous Spacers Market Outlook for Key Countries
USA Interspinous Spacers Market Analysis and Trends
The USA’s Interspinous Spacers market benefits from a high incidence of spinal disorders and robust research funding. Clinical adoption of novel spacer technologies is high, with over 70% of U.S. spinal surgery centers integrating these devices by 2025. Major companies such as Medtronic and Zimmer Biomet contribute significantly through innovative devices and aggressive market penetration strategies, sustaining the market share and revenue growth.
Germany Interspinous Spacers Market Analysis and Trends
Germany’s market is bolstered by a strong medical device manufacturing industry and favorable regulatory policies supporting spinal implant innovations. Recent data highlights a 9% annual increase in Interspinous Spacer procedures from 2023 to 2025. Companies like B. Braun Melsungen AG lead domestic innovation efforts, partnering with research institutions to develop next-generation implants, positioning Germany as a key European growth hub within this market.
Analyst Opinion
Increasing adoption of minimally invasive spinal implants is a critical quantitative indicator steering the Interspinous Spacers market revenue upwards. Recent surgical registry data from 2024 indicate over a 15% annual increase in spinous process spacer procedures within North America, reflecting higher patient preference and surgeon confidence in these devices.
The pricing dynamics of Interspinous Spacers reveal a steady decline in unit costs due to technological advancements in polymer materials and manufacturing efficiency, enhancing affordability and demand. Data from 2025 shows a 7% reduction in average device pricing compared to 2023, facilitating wider adoption across emerging markets.
From a demand-side perspective, the expanding geriatric population has led to increased prevalence of lumbar spinal stenosis, with CDC reports estimating a 12% rise in lumbar disorder treatments from 2022 through 2025 in developed economies. This directly correlates with higher Interspinous Spacer usage in clinical settings worldwide.
Export and trade data of medical implants signal a notable supply-side strength, with 2024 export volumes of spinal implant devices from key manufacturing hubs like Germany and the USA growing by approximately 10%. This has expanded the global reach of Interspinous Spacers, fostering broader market penetration and raising the overall industry share.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: |
USD 480 million |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.4% | 2032 Value Projection: |
USD 875 million |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Medtronic plc, Zimmer Biomet Holdings Inc., NuVasive, Inc., Orthofix Holdings, Inc., Globus Medical, Inc., RTI Surgical, Inc., B. Braun Melsungen AG, Stryker Corporation, Aesculap AG, Life Spine, Inc. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Interspinous Spacers Market Growth Factors
The market growth is primarily fueled by the growing prevalence of lumbar spinal stenosis tied closely to an aging global population, with WHO estimates showing people over 60 will comprise 22% of the world’s population by 2030. Technological advancements improving device design for better biomechanical stabilization and minimally invasive implantation techniques are further solidifying market dynamics. Additionally, increasing awareness about spinal disorders and corresponding treatment options through physician education programs in developed regions underpins a significant rise. Moreover, healthcare infrastructure modernization and rising investments in outpatient surgical care facilities in emerging economies expand market scope and business growth prospects.
Interspinous Spacers Market Development
In March 2024, Zimmer Biomet partnered with NeuroArC to co-develop and commercialize a new generation of interspinous spacer solutions, forming a strategic alliance aimed at accelerating innovation in minimally invasive spinal decompression. The collaboration is expected to introduce next-level spacer technologies with improved biomechanical performance and broader clinical applicability.
In July 2024, NuVasive secured a USD 100 million investment from Blackstone Growth to support the development and commercialization of its interspinous spacer portfolio. The funding is targeted toward scaling R&D, expanding manufacturing capacity, and strengthening global market penetration in dynamic stabilization and motion-preservation spine devices.
Key Players
Leading Companies of the Market
Medtronic plc
Zimmer Biomet Holdings Inc.
NuVasive, Inc.
Globus Medical, Inc.
RTI Surgical, Inc.
B. Braun Melsungen AG
Stryker Corporation
Aesculap AG
Life Spine, Inc.
Competitive strategies across major market companies emphasize aggressive product portfolio expansion and strategic partnerships. For instance, Medtronic’s launch of next-generation polymeric spacers in 2024 has bolstered its revenue streams by 14%. Meanwhile, Zimmer Biomet’s acquisition of specialized implant tech startups enhanced its R&D capabilities leading to a 10% increase in device efficacy ratings during clinical evaluations in 2025.
Interspinous Spacers Market Future Outlook
In the coming years, the market is expected to grow steadily as global aging trends elevate the prevalence of degenerative spine disorders. Technological innovations—including resorbable polymers, motion-preserving implants, and image-guided placement systems—will enhance procedural safety and broaden clinical suitability. Increasing adoption of outpatient spine centers, rising healthcare access in Asia-Pacific, and stronger reimbursement clarity are anticipated to accelerate utilization. Expanding clinical research may validate interspinous devices for additional spinal pathologies, supporting wider therapeutic positioning and sustained long-term market expansion.
Interspinous Spacers Market Historical Analysis
Historically, the interspinous spacers market emerged alongside advancements in minimally invasive spine surgery, offering an alternative to traditional laminectomy and spinal fusion. Early adoption gained momentum in the 2000s as clinical awareness of lumbar spinal stenosis increased and implant materials improved in biocompatibility and durability. Developed regions—particularly the U.S. and Europe—dominated growth due to greater diagnostic capabilities, higher surgical volumes, and progressive reimbursement policies. However, initial clinical controversies surrounding implant longevity and revision rates temporarily slowed expansion, prompting manufacturers to refine device design and procedural protocols. Over time, the accumulation of positive long-term patient outcome data helped normalize usage among spine surgeons and pain specialists.
Sources
Primary Research Interviews:
Spine Surgeons
Orthopedic Device Distributors
Neurosurgery Consultants
WHO Musculoskeletal Disease Data
CMS Reimbursement Statistics
Magazines:
Orthopedics Today
Spinal News International
Medical Device
Diagnostic Industry
Journals:
Spine Journal
Journal of Neurosurgery: Spine
European Spine Journal
Newspapers:
The New York Times (Health)
The Guardian (Healthcare)
The Economic Times (Medical Devices)
Associations:
North American Spine Society (NASS)
American Academy of Orthopaedic Surgeons (AAOS)
FDA CDRH
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients