Spine Surgery Devices Market Size and Forecast – 2025 – 2032
The Global Spine Surgery Devices Market size is estimated to be valued at USD 7.8 billion in 2025 and is expected to reach USD 13.4 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 8.1% from 2025 to 2032.
Global Spine Surgery Devices Market Overview
Spine surgery devices include spinal fusion implants, fixation devices, artificial discs, interbody cages, bone graft substitutes, robotic-assisted surgical systems, and navigation-guided instruments. These products support stabilization, decompression, disc replacement, and minimally invasive spine procedures. Innovations focus on 3D-printed titanium implants, radiolucent PEEK cages, motion-preservation technologies, and smart surgical systems that improve accuracy and recovery.
Key Takeaways
The spinal fusion devices segment dominates the market share, driven by technological innovations enhancing procedural success rates.
Meanwhile, minimally invasive spine surgery procedures are the fastest-growing subsegment due to their clinical advantages and lower healthcare costs.
Degenerative disc disease remains the leading application segment, accounting for nearly 38% of device utilization globally.
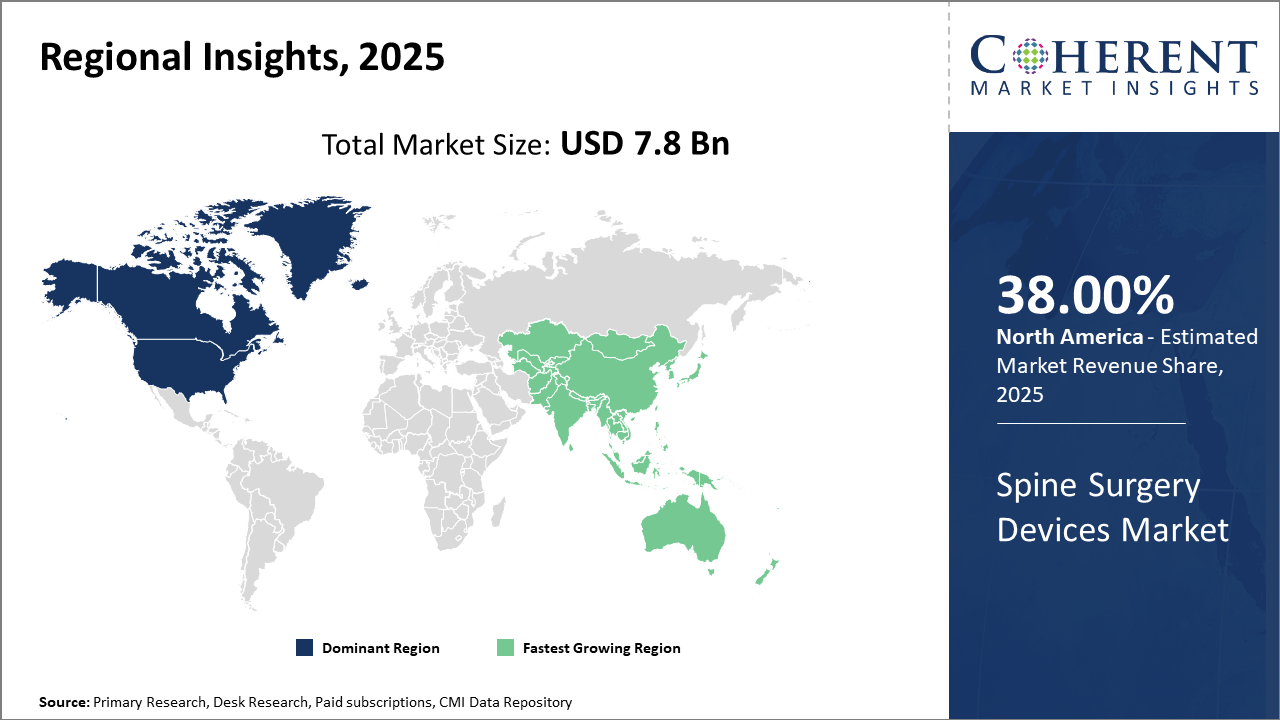
Regionally, North America holds the largest industry share at 38%, supported by high healthcare expenditure and robust infrastructure.
Asia Pacific exhibits the fastest growth, recording a CAGR above 9%, fueled by improving healthcare access and the rising incidence of spinal disorders in aging populations.
Europe’s market continues to showcase steady growth, propelled by reimbursement reforms and increasing adoption of advanced surgical technologies in countries like Germany and the U.K.
Spine Surgery Devices Market Segmentation Analysis
To learn more about this report, Download Free Sample
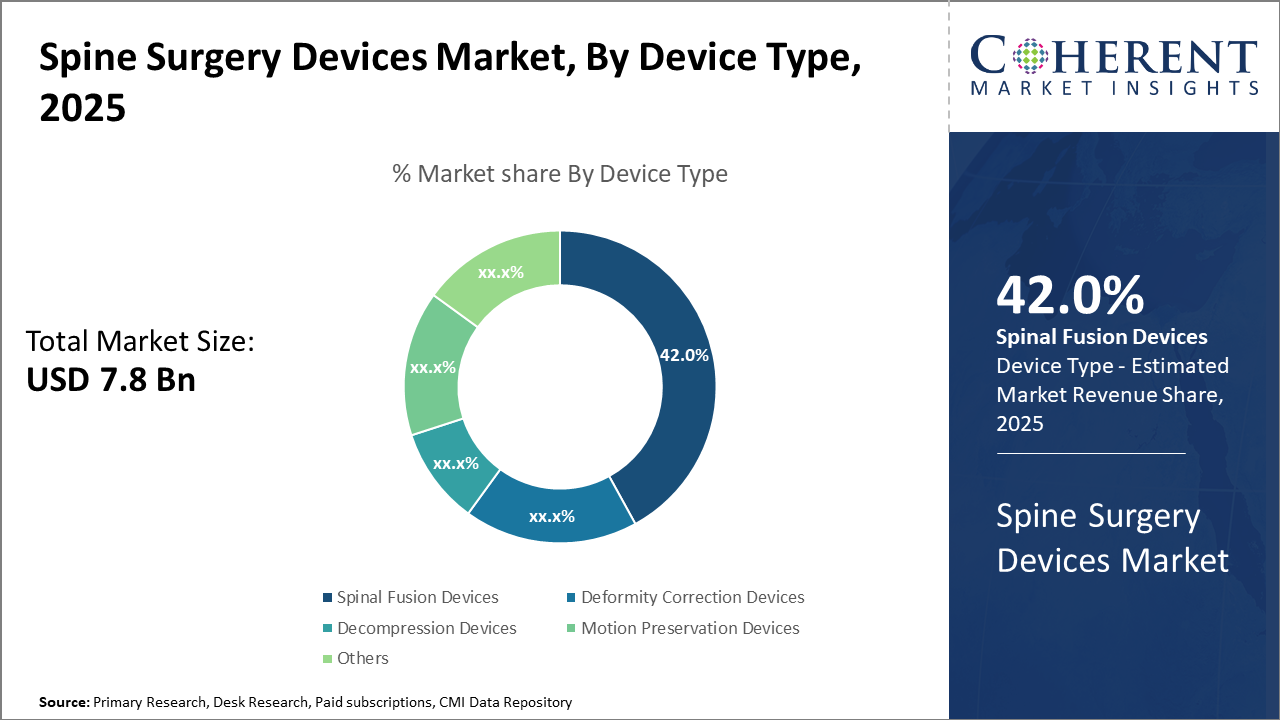
Spine Surgery Devices Market Insights, By Device Type
Spinal Fusion Devices dominate the market share at 42%. Spinal fusion devices lead due to their widespread clinical acceptance for treating degenerative disc disease and spinal trauma. The segment benefits from innovations in bioactive materials and augmented reality (AR)-assisted placement techniques, enhancing success rates and minimizing complications. The fastest-growing subsegment here is Motion Preservation Devices, propelled by increasing demand for non-fusion alternatives that maintain spinal mobility and reduce adjacent segment degeneration risks.
Spine Surgery Devices Market Insights, By Procedure Type
Minimally Invasive Spine Surgery dominating market share due to reduced patient trauma and accelerated recovery times. Growing physician expertise and greater insurance reimbursements in developed markets support this trajectory. Endoscopic Spine Surgery is the fastest growing subsegment, offering less tissue damage and better visualization capabilities that are gaining traction, particularly in the Asia Pacific region. Open Spine Surgery, although less favored due to higher complication rates and longer hospitalization, remains relevant for complex deformity corrections.
Spine Surgery Devices Market Insights, By Application
Degenerative Disc Disease commanding the largest market share, driven by its high epidemiological prevalence among aging populations worldwide. The segment benefits from continuous innovations in implant materials and biologic,s enhancing fusion rates. Spinal Trauma is the fastest-growing application, primarily due to increasing road accidents and workplace injuries in emerging economies, which have led to a surge in demand for specialized surgical equipments. Spinal Deformities represent a steady-growth segment propelled by pediatric and adult scoliosis treatment advancements.
Spine Surgery Devices Market Trends
The spine surgery devices market continues to evolve through technology-driven trends such as expansion of robotic-assisted surgery and personalized medicine.
The large-scale deployment of AI-powered surgical navigation systems in 2024 demonstrated a paradigm shift, with Medtronic and Globus Medical notably contributing to wider adoption by validating improved clinical outcomes.
The surge in demand for patient-specific implants, particularly in the Asia Pacific market, reflects a trend towards customization, improving clinical efficacy and reducing complications.
Sustainability has emerged as a key factor, with companies integrating bioresorbable materials and reducing carbon footprints during manufacturing processes, which aligns with growing environmental regulations worldwide.
Spine Surgery Devices Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Spine Surgery Devices Market Analysis and Trends
In North America, the dominance in the Spine Surgery Devices market is sustained by significant healthcare infrastructure, high patient awareness, and continual investment in new technology. The U.S. accounts for the majority share due to advanced reimbursement frameworks and a well-established base of spine specialists. The high procedural volume of over 220,000 spinal fusions annually in the U.S. underscores the region's substantial market revenue.
Asia Pacific Spine Surgery Devices Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth with a CAGR exceeding 9%, driven by accelerating healthcare infrastructure development in India and China, coupled with expanding insurance coverage. Increased investments in spine care facilities and the rising prevalence of spinal disorders contribute to this trend.
Spine Surgery Devices Market Outlook for Key Countries
USA Spine Surgery Devices Market Analysis and Trends
The U.S. market is the largest contributor to the industry size, characterized by advanced technological adoption and extensive procedural volumes. Companies like NuVasive and Medtronic have consistently pushed innovations such as robot-assisted surgeries and AI-driven spinal navigation, improving surgical outcomes and expanding market revenue. Enhanced Medicare reimbursement policies and high investment in R&D contributed to over 10% growth in spine surgery procedures by 2024, solidifying the USA's leadership position.
Germany Spine Surgery Devices Market Analysis and Trends
Germany's market benefits from a robust healthcare ecosystem and proactive government initiatives aimed at improving spinal care. Adoption of minimally invasive devices increased by 18% in 2024, supported by health insurance reforms and clinical preference shifts towards outpatient procedures. Leading companies have introduced advanced spinal fusion systems adapted to German patient demographics, bolstering market share and business growth.
Analyst Opinion
The expansion of minimally invasive spine surgery devices has been a significant supply-side driver. In 2024, global production capacity of such devices increased by over 15%, primarily fueled by higher adoption rates in ambulatory surgical centers. Pricing trends indicate a gradual reduction in implant costs, improving affordability and thereby increasing market size significantly. For instance, a shift in kyphoplasty device pricing in North America led to a 12% volume increase in 2024.
On the demand side, rising spinal fusion procedures—projected to exceed 650,000 globally in 2025—have been a pivotal growth indicator. The increasing preference for advanced navigation and robotic assistance technologies has augmented device demand across orthopedic and neurosurgical specializations. This elevated use case diversified product applications, corroborated by the 18% import growth of navigation systems in Europe in early 2025.
Micro-segmentation reveals a surge in demand from geriatric patients due to osteoporosis-related fractures and deformities—a demographic segment that accounted for nearly 28% of total procedure volumes in 2024. Improvements in biocompatible and bioresorbable device coatings catered to this niche, evidencing a 20% revenue increase for spinal implant manufacturers targeting elderly care.
Nano-scale innovations in spinal biologics and device coatings are underpinning future market dynamics. In 2025, several companies introduced nano-engineered surface treatments that enhanced osteointegration rates by 30–35%, thereby expanding clinical indications and improving patient outcomes. This advancement directly feeds into the overall upward trajectory of the Spine Surgery Devices market revenue.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: |
USD 7.8 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.1% | 2032 Value Projection: |
USD 13.4 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Medtronic, Stryker, Zimmer Biomet, NuVasive, Globus Medical, DePuy Synthes (Johnson & Johnson), Smith & Nephew, K2M, B. Braun, RTI Surgical. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Spine Surgery Devices Market Growth Factors
The growing prevalence of spinal disorders, especially degenerative diseases and trauma, remains a primary market driver, underpinned by the World Health Organization's report indicating spinal ailments as a top cause of disability globally. Advances in minimally invasive procedures significantly reduce patient recovery times and hospital stays, promoting adoption across emerging healthcare systems, notably in the Asia Pacific. An aging population drives consistent demand for spine surgery devices, with demographic studies highlighting a 15% increase in spinal surgery volume for patients aged above 65 in 2024. Furthermore, reimbursement policy enhancements in developed countries have incentivized hospital systems to invest in advanced surgical solutions, as observed in the U.S. Medicare expenditures for spine fusion procedures rising by 10% year-over-year.
Spine Surgery Devices Market Development
In November 2023, Orthofix Medical Inc. launched the WaveForm® L Lateral Lumbar Interbody System, a 3D-printed interbody implant designed for lateral lumbar interbody fusion (LLIF). The device features a porous, wave-structure “body” to improve load distribution, graft retention, and bone ingrowth.
In December 2024, Carlsmed received FDA 510(k) clearance for its aprevo® Cervical ACDF Interbody System, marking a major step in its “personalized spine surgery” strategy. The device is designed using AI-assisted planning and custom manufacturing to match patient-specific anatomy. Commercial launch is targeted for 2025
Key Players
Leading Companies of the Market
Medtronic
Stryker
Zimmer Biomet
NuVasive
Globus Medical
DePuy Synthes (Johnson & Johnson)
Smith & Nephew
K2M
B. Braun
RTI Surgical, among others.
Several leading market players have strategically focused on launching next-generation spinal implants integrated with robotic-assisted surgical platforms. For instance, Medtronic’s investment in robot-assisted navigation solutions facilitated a 25% increase in spinal procedure efficiency in 2024. Business growth strategies such as strategic acquisitions and collaborations have been pivotal. Zimmer Biomet's 2024 acquisition of a pioneering AI-driven spine analytics firm expanded its portfolio and accelerated market share growth by 8% within a year.
Spine Surgery Devices Market Future Outlook
The spine market will converge around personalized implants, smarter instrumentation, and value-based pathways. Expect growth in patient-matched 3D-printed implants with porous architectures for bone in-growth, bioactive coatings to accelerate fusion, and expandable devices that simplify endplate preparation. Robotic systems and AI-assisted planning will further refine trajectory planning and reduce intraoperative variability. Minimally invasive and ambulatory spine procedures will expand with lower-profile implants and improved hemostatic systems. Health systems will increasingly require health-economics evidence (reduced LOS, fewer revisions) which will shape adoption timelines; integration of outcome registries and surgical analytics will become standard for competitive product claims.
Spine Surgery Devices Market Historical Analysis
Spine surgery devices evolved from rudimentary fixation plates and bone grafts into a complex ecosystem of implants, biologics and digital technologies. The early era emphasized open fusion with metal instrumentation; over time, interbody cages, pedicle screw-rod constructs, and bone graft substitutes became standard. The 1990s–2010s saw major shifts: minimally invasive approach instruments, expandable cages, and porous/titanium 3D-printed implants improved fusion rates and reduced morbidity. Concurrently, surgical navigation, intraoperative imaging, and later robotic assistance enhanced placement accuracy and reduced revision rates. Increased focus on motion-preserving technologies spurred disc arthroplasty development, while biologics and bone morphogenetic proteins opened new frontiers for promoting osseointegration—albeit with regulatory and safety scrutiny.
Sources
Primary Research Interviews:
Orthopedic spine surgeons
Neurosurgeons
Implant R&D engineers
Hospital procurement directors
Databases:
PubMed Spine Research
FDA 510(k) & PMA databases
ClinicalTrials.gov (spine device trials)
National Joint & Spine Registries
Magazines:
Spine (OREF News)
Becker’s Spine Review
Orthopedics This Week
Medical Device + Diagnostic Industry (MD+DI)
Journals:
The Spine Journal
Journal of Neurosurgery: Spine
European Spine Journal
Newspapers:
The Wall Street Journal (Healthcare)
Financial Times (MedTech)
The Hindu (Health)
The New York Times (Science)
Associations:
North American Spine Society (NASS)
American Association of Neurological Surgeons (AANS)
European Spine Society (EuroSpine)
FDA CDRH
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients