Global Implantable Medical Devices Market Overview
In order to replace a missing biological structure, sustain a damaged biological structure, or improve an existing biological structure, implantable medical devices are man-made devices that are put inside or on the body. Through surgical procedures, implantable medical devices are assisting numerous people in improving their quality of life. The implants can be used in different body parts for numerous applications such as pacemakers, orthopaedics, cardiovascular stents, neural prosthetics or drug delivery system, and defibrillators. Depending on the intended compatibility and use, biomedical materials like silicone, titanium, or another substance make up the surface of implantable medical devices. When no longer required, implantable medical devices might be taken out or left in place permanently.
Implantable Medical Devices Market Size and Forecast – 2025 – 2032
The Implantable Medical Devices Market size is estimated to be valued at USD 115.6 billion in 2025 and is expected to reach USD 178.2 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 6.1% from 2025 to 2032.
Key Takeaways
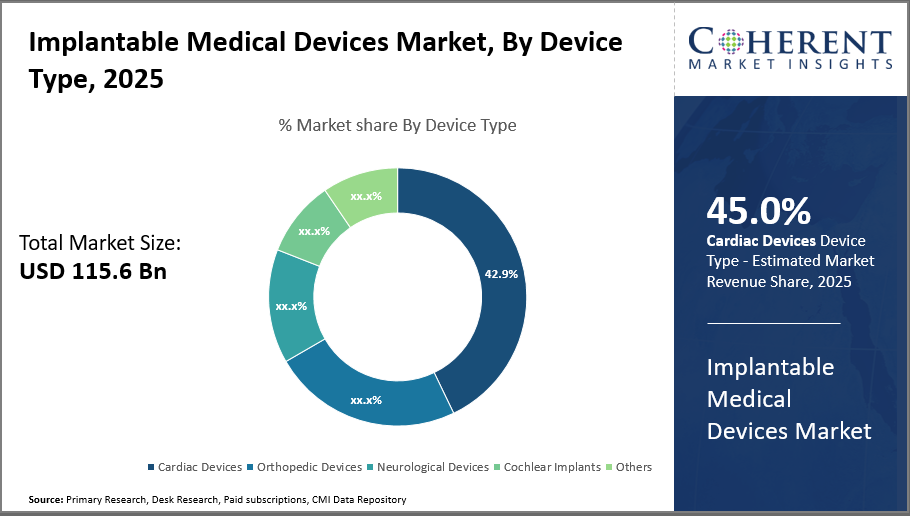
In terms of device type, cardiac devices dominate implantable medical devices market share with 45%, attributable to the high incidence of cardiovascular disorders globally.
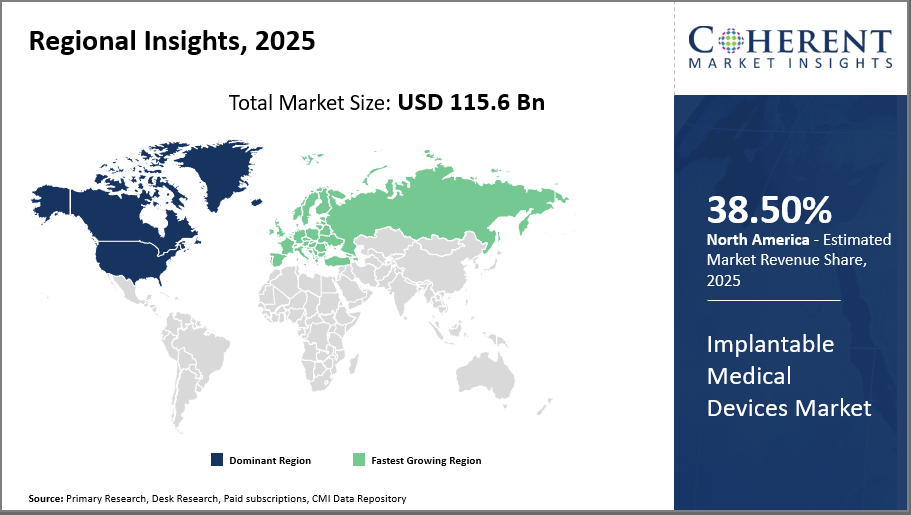
Based on regional insights, North America leads market share with 38.5%, buoyed by advanced healthcare infrastructure and reimbursement mechanisms, while Asia Pacific exhibits the highest CAGR driven by expanding healthcare investments and government support.
Europe maintains steady growth with growing adoption of neurological devices in chronic disease management protocols. These key takeaways underline vital segments and regions shaping ongoing market trajectory.
Implantable Medical Devices Market – Segmentation Analysis
To learn more about this report, Download Free Sample
Implantable Medical Devices Market Insights, By Device Type
The market is divided into several segments based on the kind of device, including Cardiac Devices, Orthopedic Devices, Neurological Devices, Cochlear Implants, And Others. Of these, cardiac devices hold a dominant market share of 45%. The rising incidence of cardiovascular disease and ongoing advancements like implantable cardioverter defibrillators and leadless pacemakers, which improve therapy safety and effectiveness, are advantageous to cardiac devices.
Implantable Medical Devices Market Insights, By Application
In terms of Application, the market is segmented into Cardiovascular, Orthopedic, Neurological, and Others, with Cardiovascular application leading with the largest market share. The upsurge in heart diseases worldwide compels extensive use of implantable devices such as stents and pacemakers, accounting for significant market revenue.
Implantable Medical Devices Market Insights, By End-User
Hospitals & Clinics, Ambulatory Surgical Centers, Home Healthcare, and Others make up the market's end-user segments, with hospitals and clinics holding a dominant market share. The extensive use of complex implantable devices is made possible by hospitals' access to cutting-edge surgical equipment and skilled staff.
Implantable Medical Devices Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Implantable Medical Devices Market: An Analysis and Trends
Having a significant 38.50% market share in North America, the market for implantable medical devices is driven by a robust healthcare system, broad insurance coverage, and the quick uptake of new technologies. With significant R&D expenditures and government programs encouraging the use of cardiac and neurological devices, the United States is the key hub. The presence of major industry participants like Abbott Laboratories and Medtronic reinforces this dominance by actively supporting regional market revenue and personalized medicine-focused growth initiatives.
Europe Implantable Medical Devices Market: An Analysis and Trends
The aging population, growing prevalence of chronic diseases, and growing use of minimally invasive therapies are all contributing factors to the stable expansion of the European implantable medical devices market. Germany is the market leader because of its sophisticated healthcare system, concentration on R&D, and quick technical development. Orthopedic, cardiovascular, and dental implants are important product categories; polymers are preferred for their biocompatibility and flexibility. The increasing use of implanted devices, which enhance patient outcomes and lessen the need for repeated interventions, is supported by the European healthcare focus on value-based care.
Implantable Medical Devices Market Outlook for Key Countries
Market Analysis and Trends for Implantable Medical Devices in the United States
About 30% of the market revenue for implantable medical devices comes from the US, making it a crucial market. The newest pacemakers and neurostimulators are among the many devices that are continuously introduced thanks to technological leadership, comprehensive clinical trials, and advantageous reimbursement schemes. Businesses that make significant investments in clinical validation and innovation, such as Boston Scientific and Medtronic, propel adoption in the neurology and cardiology sectors.
Market Analysis and Trends for Implantable Medical Devices in the Germany
Germany has one of the biggest markets for implantable medical devices in Europe, thanks to a strong medical technology industry and an established healthcare system. Strong market participants that concentrate on cardiac and orthopedic implants include B. Braun and Biotronik. Favorable conditions for market expansion are created by government programs that prioritize digital health and increase spending on managing chronic diseases.
Analyst Opinion
Rising Production Capacities and Innovation Pipelines: Manufacturing advancements have led to a 12% increase in production capacity for implantable devices in 2024, with significant investments funneled into R&D initiatives aimed at enhancing device miniaturization and longevity. For instance, a notable increase in FDA approvals for cardiac implants demonstrates a growing supply-side readiness pushing market size expansion.
Expanding Clinical Applications Sparking Demand-side Growth: The diversification of use cases, such as neural stimulators for chronic pain management, expanded by 15% in procedural volume during 2025, indicating rising import and adoption rates. Hospitals globally are integrating these devices across new therapeutic domains, contributing to market revenue growth and higher industry penetration.
Enhanced Pricing Models Reflecting Market Dynamics: An observed 5% year-over-year price stabilization in high-demand orthopedic implants reflects market players' efforts to balance affordability with innovation costs, aiding in sustained business growth despite economic fluctuations.
Regional Demand Shifts Influencing Market Share: North America accounted for approximately 38.5% of the implantable medical devices market share in 2024, supported by robust healthcare infrastructure and reimbursement policies. Emerging markets in Asia Pacific have seen device adoption grow over 20% annually, driven by government initiatives targeting chronic illness management and growing medical infrastructure.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 115.6 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.1% | 2032 Value Projection: | USD 178.2 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Medtronic plc, Boston Scientific Corporation, Abbott Laboratories, Stryker Corporation, B. Braun Melsungen AG, Siemens Healthineers, Cochlear Limited, Zimmer Biomet Holdings, Inc., Edwards Lifesciences Corporation, Biotronik SE & Co. KG | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Growth factors
Key factors driving the implantable medical devices market include the increasing prevalence of cardiovascular diseases, which remain the leading cause of mortality globally, with over 18 million deaths reported in 2024. Technological advancements in minimally invasive implant procedures have expanded the target patient demographic, significantly contributing to enhanced market growth.
Furthermore, rising healthcare expenditure, especially in regions like North America and Europe, alongside government-led reimbursement policies, has improved market access and device affordability. Lastly, the integration of IoT and AI with implantable devices has driven demand for smart and connected devices, a trend with a reported CAGR of 28% in device connectivity from 2023 to 2025, facilitating better patient monitoring and clinical outcomes.
Implantable Medical Devices Market Development
Market Trends
The implantable medical devices market is witnessing significant shifts towards enhanced digitalization, with smart and connected devices leading the trend. Wireless charging and remote monitoring capabilities, increasingly adopted in 2024, have improved patient compliance and reduced hospitalization durations.
Additionally, biodegradable implants are becoming more popular; preliminary studies have shown a 25% decrease in post-operative problems. Following the discovery of more vulnerabilities in medical IoT, regulatory attention is shifting to cybersecurity guidelines for linked devices. Regional changes are also noteworthy; because of cost savings and advantageous regulations, Asia Pacific is quickly becoming a centre for the production of cutting-edge devices, changing the dynamics of the conventional market.
Key Players
Medtronic plc
Boston Scientific Corporation
Stryker Corporation
B. Braun Melsungen AG
Siemens Healthineers
Cochlear Limited
Zimmer Biomet Holdings, Inc.
Edwards Lifesciences Corporation
Biotronik SE & Co. KG
Competitive strategies have included focused product innovation and strategic partnerships. Medtronic's collaboration with key tech firms on next-gen implantable pacemakers resulted in 8% market share growth across North America. Similarly, Johnson & Johnson’s acquisition of innovative neurotechnology start-ups enhanced its portfolio reach, improving penetration in emerging regions by nearly 12% in 2024.
Implantable Medical Devices Market Future Outlook
The market for implantable medical devices has a bright future because of the rising incidence of chronic illnesses and the aging of the population, which increases demand for cutting-edge implantable solutions. It is anticipated that innovations like bioresorbable materials, smart implants, and the incorporation of artificial intelligence would improve patient outcomes and device functionality. By lowering recovery periods and difficulties, the trend toward less invasive operations are increasing the usage of implantable devices.
Strong healthcare infrastructure, rising healthcare spending, and pro-business government policies are expected to propel expansion in geographic regions like North America, Europe, and Asia Pacific. Because of increased awareness and easier access to healthcare, emerging nations are also starting to make significant contributions. Technological developments and more R&D expenditures are anticipated to support strong market expansion in the upcoming years, notwithstanding obstacles such high costs and regulatory barriers. All things considered, the market for implantable medical devices is expected to increase steadily in the future due to ongoing advancements in patient-centred solutions, connectivity, and device design.
Historical Analysis
The analysis of the market for implantable medical devices throughout time showed consistent increase, mostly due to the rising incidence of chronic illnesses such as diabetes, arthritis, cardiovascular disease, and neurological disorders. An important factor in the growing need for orthopedic, cardiovascular, dental, and neurological implants was the aging of the world's population. Technological developments that improved patient outcomes and expanded the uses of implantable devices, such as miniaturization, digital health integration, and the creation of intelligent devices, helped the market in recent years.
The United States emerged as a major market due to high healthcare expenditure, advanced infrastructure, and supportive regulatory frameworks. Europe also demonstrated considerable growth, driven by a strong healthcare system and increasing chronic disease cases. Meanwhile, the Asia Pacific region rapidly expanded its market share due to increased healthcare investments, improving infrastructure, and rising awareness. The market landscape included a mix of established global players and emerging companies focused on innovation and strategic partnerships.
Sources
Primary Research interviews:
Neurologists
Dental surgeons
Biomedical engineers
Databases:
Web of Science
ClinicalTrials.gov
Medline
Magazines:
MedTech Outlook
DeviceMed
MPO (Medical Product Outsourcing)
Journals:
Biomaterials
Nature Biomedical Engineering
Journal of Medical Devices (ASME)
Newspapers:
The Wall Street Journal – Health & Science
The Economic Times – Healthcare
The Hindu – Health
Associations:
Medical Device Manufacturers Association (MDMA)
European Coordination Committee of the Radiological,
Electromedical and Healthcare IT Industry (COCIR)
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients