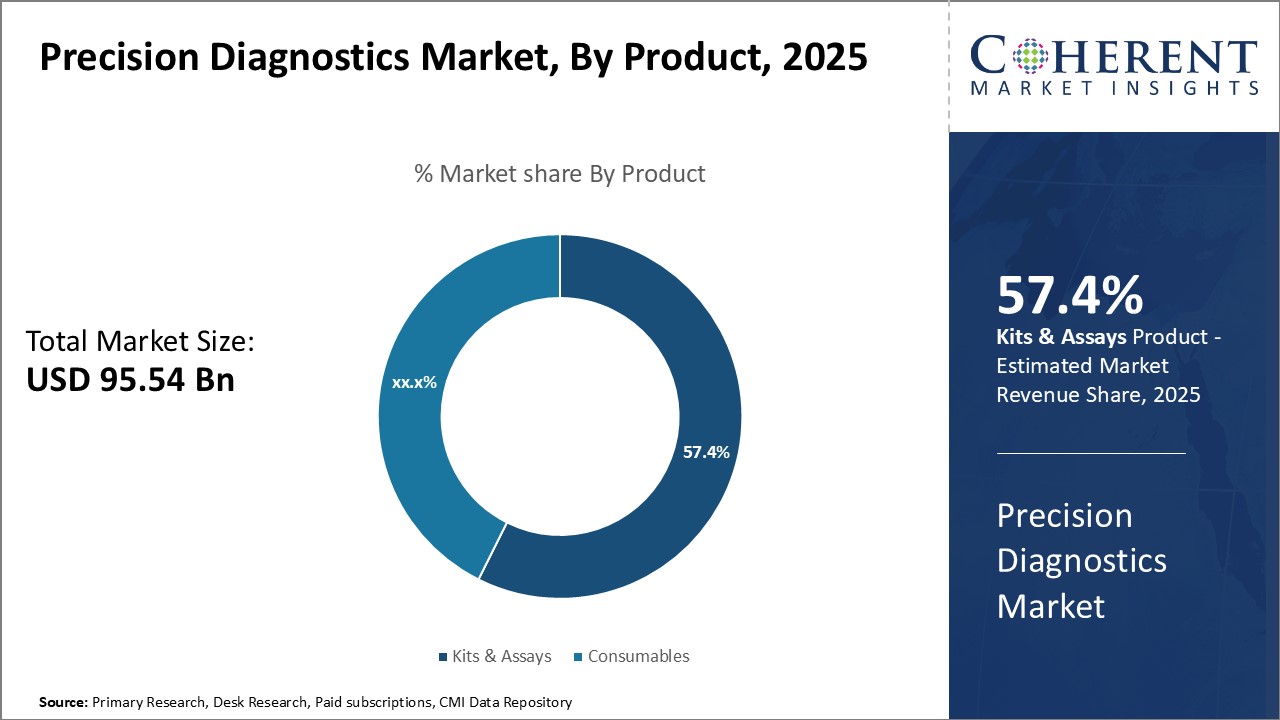
The precision diagnostics market is estimated to be valued at USD 95.54 Bn in 2025 and is expected to reach USD 224.90 Bn by 2032, growing at a compound annual growth rate (CAGR) of 13.0% from 2025 to 2032.
To learn more about this report, Download Free Sample
Key Takeaways:
- By Product, The Kits & Assays segment is projected to dominate the global precision diagnostics market, capturing approximately 57.4% of the market share in 2025.
- By Technology, The Polymerase Chain Reaction (PCR) technology segment is expected to lead the market, accounting for 34.3% of the market share in 2025.
- By Application, Early Detection and Intervention division is projected to be the most significant application area, holding 30.1% of the market share in 2025.
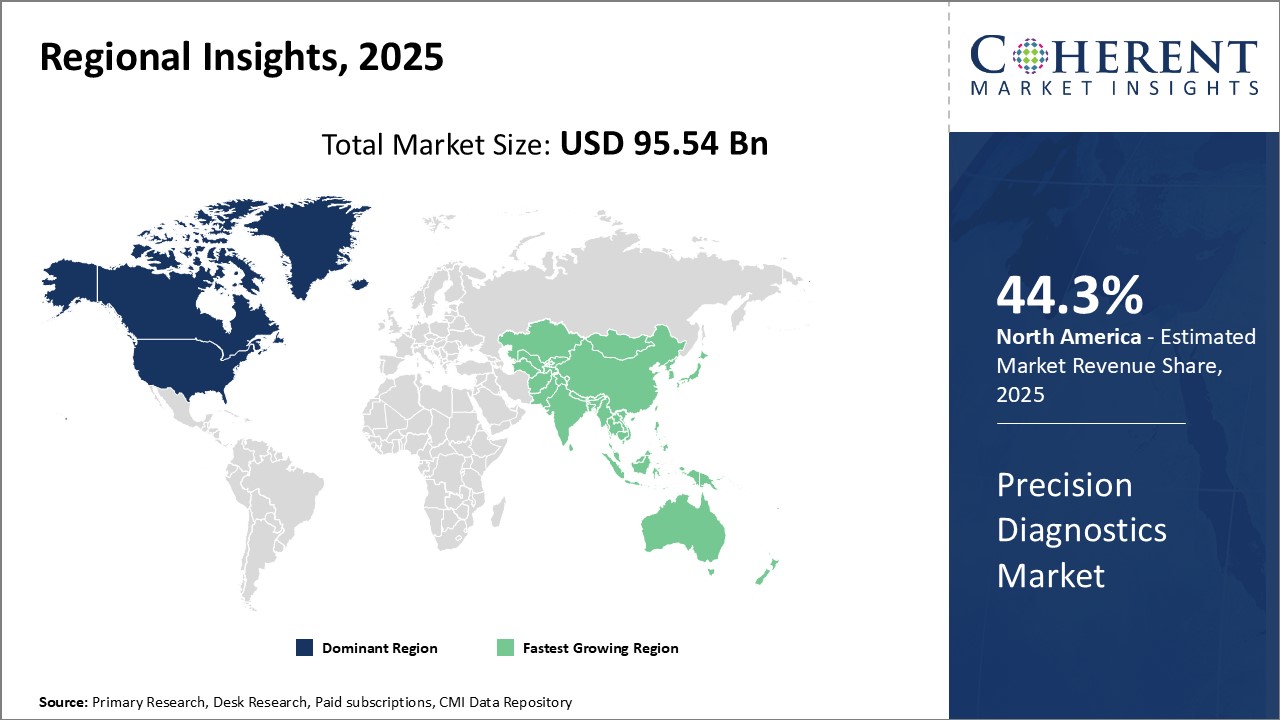
- North America is anticipated to maintain a dominant role in the global precision diagnostics market, capturing 44.3% of the market share in 2025.
Market Overview:
The global Precision Diagnostics Market is experiencing robust growth, driven by the rising demand for accurate, personalized, and early-stage disease detection across healthcare systems worldwide. The market is significantly benefiting from technological advancements in genomics, molecular diagnostics, and bioinformatics, which are enhancing diagnostic accuracy and enabling tailored treatment strategies.
Current Events and its Impact on the Precision Diagnostics Market
|
Current Event |
Description and its impact |
|
Rise in Demand for Personalized Healthcare |
|
|
Technological Advancements in Molecular Diagnostics |
|
|
Regulatory and Policy Support for Genomic Medicine |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Pipeline Analysis: Precision Diagnostics Market
The pipeline for the precision diagnostics market is robust, marked by a surge in advanced diagnostic technologies targeting oncology, infectious diseases, and genetic disorders. Key players are actively developing next-generation sequencing (NGS) platforms, liquid biopsy assays, and companion diagnostics tailored for personalized treatment.
Numerous clinical trials are underway focusing on multi-analyte blood tests for early cancer detection, AI-powered diagnostic algorithms, and CRISPR-based molecular tools. Companies like Roche, Illumina, and Thermo Fisher Scientific are expanding their R&D portfolios, often collaborating with biotech startups and research institutes to accelerate innovation.
Additionally, regulatory agencies such as the FDA are offering expedited approval pathways for breakthrough diagnostics, encouraging faster market entry. The growing emphasis on preventive care and personalized medicine is fueling investment in early-stage research and commercialization strategies. As a result, the pipeline is expected to deliver a steady influx of transformative diagnostic products over the next 3–5 years, reshaping patient care outcomes globally.
Patent Landscape: Precision Diagnostics Market
The precision diagnostics market exhibits a dynamic and competitive patent landscape, reflecting the rapid pace of innovation in molecular diagnostics, biomarker discovery, and genomic technologies.
Major players such as Roche, Abbott Laboratories, Thermo Fisher Scientific, and Illumina hold a significant number of patents covering advanced diagnostic platforms, including polymerase chain reaction (PCR), next-generation sequencing (NGS), and companion diagnostics. These patents often focus on novel assay formulations, detection techniques, and proprietary algorithms for analyzing genetic and proteomic data.
Additionally, emerging startups and research institutions are actively filing patents related to AI-driven diagnostics, liquid biopsy technologies, and point-of-care testing solutions. The U.S. and Europe lead in patent filings, with increasing activity also observed in China and Japan. The patent landscape is further shaped by strategic collaborations and licensing agreements, enabling access to critical technologies while reducing time-to-market.
Reimbursement Scenario: Precision Diagnostics Market
The reimbursement landscape for precision diagnostics is evolving, marked by both progress and persistent challenges. In high-income regions like North America and Western Europe, reimbursement frameworks are increasingly accommodating advanced diagnostics, especially in oncology and rare disease applications.
For instance, the Centers for Medicare & Medicaid Services (CMS) in the U.S. has proposed separate reimbursement for high-cost diagnostic radiopharmaceuticals, such as Lantheus' Pylarify, enhancing access to specialized tests.
Conversely, in low- and middle-income countries, inadequate reimbursement policies hinder the adoption of precision diagnostics. The high costs associated with these tests, coupled with limited insurance coverage, restrict their accessibility, particularly in Latin America and parts of Asia and Africa.
Additionally, the regulatory environment is becoming more stringent. The FDA's 2023 mandate requiring authorization for laboratory-developed tests (LDTs) aims to ensure test accuracy but may increase development costs and impact reimbursement dynamics.
Prescribers’ Preference: Precision Diagnostics Market
Prescribers are increasingly favoring precision diagnostics due to their ability to offer targeted, data-driven insights that enhance clinical decision-making. In 2025, oncologists, geneticists, and primary care physicians are leading the adoption of these tools, particularly in areas such as cancer, rare diseases, and pharmacogenomics.
The preference is driven by the high accuracy, early detection capabilities, and personalized treatment guidance that precision diagnostics provide. Tests such as next-generation sequencing (NGS), companion diagnostics, and liquid biopsies are gaining popularity, enabling clinicians to select the most effective therapies based on a patient’s genetic profile.
Additionally, prescribers value the reduced trial-and-error approach and improved patient outcomes linked to these diagnostics. However, uptake varies based on awareness, training, and availability of infrastructure, with practitioners in urban and developed healthcare settings showing higher preference.
Market Concentration and Competitive Landscape

To learn more about this report, Download Free Sample
Precision Diagnostics Market Trends
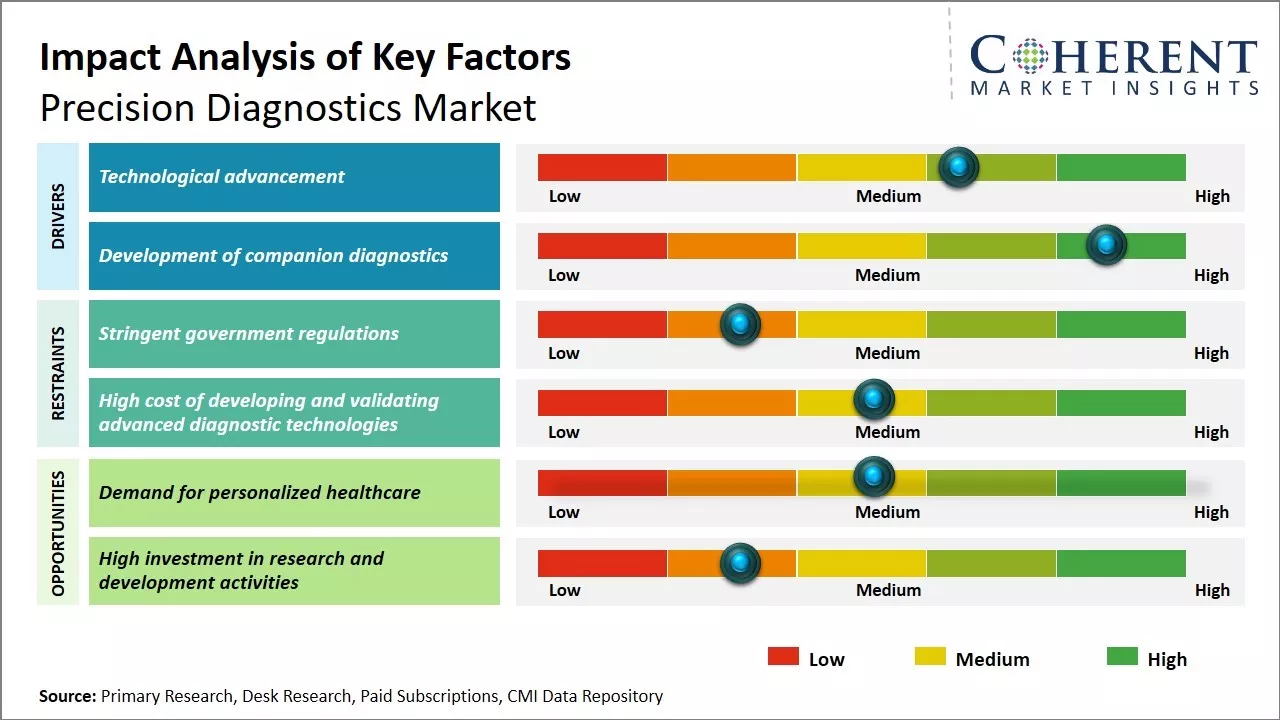
- Technological advancement
The precision diagnostics market is being driven significantly by the rapid advancements happening in technologies being used for diagnostics. New and innovative diagnostic tools that leverage cutting edge technologies are allowing for much more accurate diagnosis of diseases. Technologies, such as next generation sequencing, laboratory automation, machine learning, and artificial intelligence, are enabling precision diagnostics to reach new levels.
Next generation sequencing has revolutionized genetic testing and enabled the diagnosis of various inherited disorders, cancers, and infections that were not possible to detect earlier with traditional methods. For instance, in May 2020, Paragon Genomics, a precision medicine company based in the U.S., announced a partnership with Saphetor, a Switzerland-based precision medicine company.
Paragon Genomics' ready-to-use and custom NGS panel solutions have enabled several lab-developed tests (LDTs) in infectious illnesses, oncology, reproductive health, cardiology, and genetic diseases across the globe. In February 2024, Intel announced the official launch of Altera as a standalone FPGA (Field-Programmable Gate Array) company, marking a strategic move to intensify its focus on FPGA development. This restructuring aims to boost agility in product development and better cater to high-growth sectors such as artificial intelligence, automotive, and aerospace.
- Development of companion diagnostics
Another major factor bolstering growth in the precision diagnostics market is the increasing integration of companion diagnostics with targeted therapies. Companion diagnostics are in vitro diagnostic tests or imaging tools that provide critical information for the safe and effective use of corresponding therapies or drugs. They help identify patients that are most likely (or not likely) to benefit from specific treatments. This allows for more precise selection of treatment options for patients and prevents potential adverse reactions.
Regulatory agencies now mandate the development and approval of companion diagnostics along with several new targeted therapies. This is driving significant R&D investments from pharmaceutical companies into precision diagnostics. For instance, most new oncology drugs are now launched along with a corresponding DNA or protein-based companion diagnostic test to identify patients with specific genomic alterations or biomarkers.
In early 2025, NeoGenomics, a leading provider of cancer-focused genetic testing services, announced optimistic financial projections, anticipating revenues between $735 million and $745 million for the year. This outlook reflects the company's confidence in the expanding demand for precision oncology diagnostics. NeoGenomics also envisions long-term growth, with plans to serve over 1 million patients annually by 2028.
These projections come amid a leadership transition, as CEO Chris Smith announced his retirement. Despite this change, the company’s strategic focus on innovation and partnerships, such as its collaboration with Adaptive Biotechnologies, positions it to capitalize on advancements in personalized cancer care.
Opportunities in the precision diagnostics Market
The growing need for personalized healthcare tailored to individual patient genotypes and disease subtypes is driving demand for precision diagnostics kits. Genomic sequencing and other advanced techniques are enabling new diagnosis and treatment strategies. Demand for non-invasive liquid biopsies using blood or other bodily fluids is increasing rapidly, thus creating lucrative growth opportunities for market growth over the forecasted period.
Precision Diagnostics Market Insights, By Product
The Kits & Assays segment is projected to dominate the global precision diagnostics market, capturing approximately 57.4% of the market share in 2025. This leading position can be attributed to continuous technological innovations that enhance the speed, accuracy, and reliability of diagnostic outcomes. Kits & assays are preferred in both clinical and research settings due to their simplicity, rapid turnaround times, and compatibility with point-of-care and decentralized testing platforms.
Precision Diagnostics Market Insights, By Technology
The Polymerase Chain Reaction (PCR) technology segment is expected to lead the precision diagnostics market in 2025, holding 34.3% of the market share. PCR remains a cornerstone of molecular diagnostics due to its high sensitivity, specificity, and rapid results. Its widespread application in infectious disease rapid diagnostics, oncology, and genetic testing underpins its sustained leadership.
Precision Diagnostics Market Insights, By Application:
The Early Detection and Intervention segment is projected to be the most significant application area, capturing 30.1% of the market share in 2025. This dominance is driven by growing awareness of the clinical and economic benefits of early disease identification, particularly for chronic and life-threatening conditions such as cancer and genetic disorders.
Regional Insights
To learn more about this report, Download Free Sample
North America Precision Diagnostics Market Trends and Analysis
North America is anticipated to maintain a dominant role in the global precision diagnostics market, capturing 44.3% of the market share in 2025. This regional leadership is supported by the strong presence of leading diagnostics companies, advanced healthcare infrastructure, and substantial R&D investments. The United States continues to be a global hub for precision medicine initiatives, with supportive government policies, high adoption of cutting-edge diagnostic technologies, and rising demand for early disease detection.
Europe Precision Diagnostics Market Trends and Analysis
Europe is expected to remain a key region in the precision diagnostics market through 2025, driven by a strong focus on personalized healthcare, particularly in countries such as Germany, the UK, and France. The region benefits from well-structured national healthcare systems, increasing public and private sector investments in molecular diagnostics, and active participation in EU-funded genomic research programs.
Precision Diagnostics Market Dominating Countries:
United States and Canada Precision Diagnostics Market Analysis and Trends
The United States and Canada dominate the North American precision diagnostics market, which is expected to account for 44.3% of the global market share in 2025. The U.S. leads with its advanced healthcare infrastructure, substantial government funding for precision medicine initiatives, and the presence of major diagnostics companies such as Thermo Fisher Scientific, Illumina, and Danaher.
Robust regulatory support from the FDA and NIH-backed research programs are accelerating the development and adoption of precision diagnostics. Canada contributes through a well-coordinated public healthcare system, growing investments in genomics, and initiatives like Genome Canada that support innovative diagnostic research and deployment.
Market Report Scope
Precision Diagnostics Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 95.54 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 13.0% | 2032 Value Projection: | USD 224.90 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
SomaLogic, Ezra, Egnite, PrecisionLife, HALO Precision Diagnostics, Danaher Corporation, QuidelOrtho Corporation, Precision Diagnostics, Koninklijke Philips N.V., , Swiss Precision Diagnostics GmbH, Precision Diagnostic Laboratory, Abbott, Cepheid, Hologic, Inc. , Precision Medicine Group, LLC. , Thermo Fisher Scientific, and Illumina, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Analyst Viewpoint – Precision Diagnostics Market
Analysts view the precision diagnostics market as a high-growth sector driven by technological innovation and an increasing focus on personalized medicine. The integration of advanced molecular techniques like next-generation sequencing, digital PCR, and AI-driven data analytics is expected to transform diagnostic accuracy and speed.
Analysts highlight the expanding applications in oncology, infectious diseases, and genetic disorders as key growth catalysts. Furthermore, regulatory support and rising healthcare expenditure in developed regions bolster market expansion. However, challenges such as high costs, complex reimbursement policies, and limited access in emerging economies may temper growth.
Analysts emphasize the importance of strategic partnerships, mergers, and collaborations to accelerate product development and market penetration. Overall, the outlook remains positive, with expectations that precision diagnostics will become a standard component of clinical workflows, significantly improving patient outcomes and enabling more cost-effective healthcare delivery worldwide.
Precision Diagnostics Market: Key Development
- In May 2025, Roche announced the launch of its updated cobas® pro integrated solutions platform, featuring enhanced automation and faster throughput for precision diagnostics in high-volume laboratories. This advancement supports clinical efficiency and strengthens Roche’s leadership in molecular and immunoassay diagnostics.
- In April 2025, Thermo Fisher Scientific partnered with Illumina to co-develop NGS-based companion diagnostics for oncology. This strategic collaboration aims to advance personalized cancer treatment by integrating high-throughput genomic technologies into clinical decision-making.
- In March 2025, Qiagen launched its QIAstat-Dx Analyzer 2.0, a next-generation syndromic testing platform with expanded panels for infectious diseases and antimicrobial resistance detection. This innovation enhances diagnostic accuracy and speed at the point of care.
- In February 2025, Siemens Healthineers received FDA clearance for its AI-powered Atellica CI Analyzer, enabling faster and more precise biomarker testing in cardiovascular and metabolic disease diagnostics.
- In January 2025, Bio-Rad Laboratories acquired Curiosity Diagnostics to accelerate development of its microfluidics-based molecular diagnostics platform, reinforcing its focus on rapid, high-sensitivity testing in decentralized healthcare settings.
Key Players Insights
- SomaLogic
- Ezra
- Egnite
- PrecisionLife
- HALO Precision Diagnostics
- Danaher Corporation
- QuidelOrtho Corporation
- Precision Diagnostics
- Koninklijke Philips N.V.,
- Swiss Precision Diagnostics GmbH
- Precision Diagnostic Laboratory
- Abbott
- Cepheid
- Hologic, Inc.
- Precision Medicine Group, LLC.
- Thermo Fisher Scientific
- Illumina, Inc.
Sources
The Stakeholders Consulted:
• Clinical pathologists and laboratory directors
• Medical device manufacturers and suppliers of diagnostic platforms
• Healthcare IT professionals and bioinformatics experts
• Regulatory affairs consultants and compliance officers
• Hospital procurement managers and diagnostic service providers
• Academic researchers in molecular biology, genomics, and proteomics
• Public health agencies and diagnostic policymakers
• End-users across sectors such as hospitals, reference laboratories, academic medical centers, and point-of-care settings
Databases Opened:
• U.S. Food and Drug Administration (FDA) – IVD and PMA Approval Database
• Centers for Medicare & Medicaid Services (CMS) – CLIA & Reimbursement Data
• World Health Organization (WHO) – Global Health Observatory
• National Institutes of Health (NIH) – Genomic Medicine and Precision Health Programs
Magazines & Trade Publications:
• Clinical Lab Products Magazine
• Diagnostics World News
• BioSpectrum
• Genetic Engineering & Biotechnology News (GEN)
• MedTech Dive
Scientific and Industry Journals:
• The Lancet – Diagnostics and Molecular Medicine Sections
• Nature Reviews Genetics
• Journal of Molecular Diagnostics
• Clinical Chemistry
• Precision Medicine Journal
Newspapers & Media Outlets:
• Bloomberg – Healthcare and Biotech Innovation
• Reuters – Medical Devices and Diagnostics News
• The Wall Street Journal – Health & Science
• The Economic Times – Healthcare & Pharmaceuticals
• Business Standard – Medical Technology and Innovation
Associations and Regulatory Bodies:
• U.S. Food and Drug Administration (FDA)
• Centers for Disease Control and Prevention (CDC) – Division of Laboratory Systems
• European Medicines Agency (EMA)
• College of American Pathologists (CAP)
• International Federation of Clinical Chemistry and Laboratory Medicine (IFCC)
• Indian Council of Medical Research (ICMR)
Public Domain Sources:
• World Health Organization (WHO) – Diagnostic Innovation Reports
• U.S. National Library of Medicine – PubMed and ClinicalTrials.gov
• OECD – Health Statistics and Diagnostic Innovation Reports
• European Commission – Medical Devices and In Vitro Diagnostics Policy Briefs
• National Health Service (UK) – Diagnostic Services Reports
Proprietary Research Elements:
• CMI Data Analytics Tool
• Proprietary CMI Repository of Market Data (covering past 8 years)
• CMI Expert Interviews and Transcripts (focused on diagnostics, genomics, and clinical workflow integration)
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients