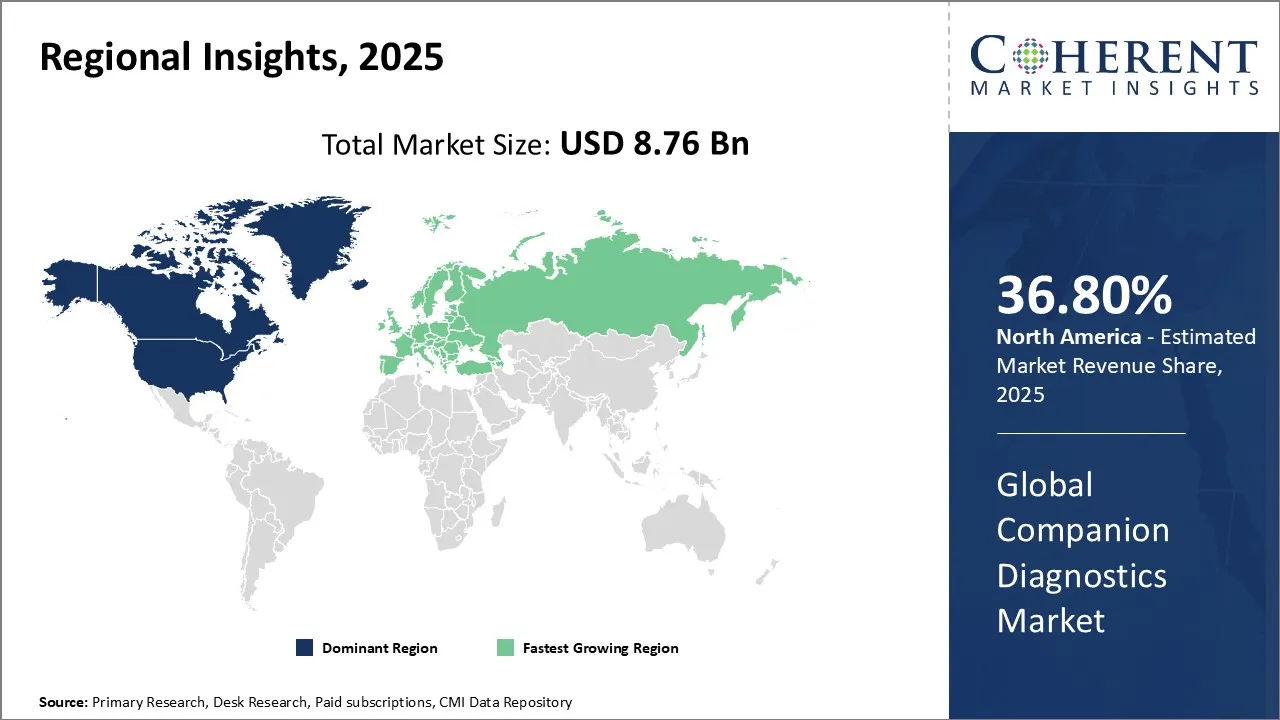
Companion Diagnostics Market is estimated to be valued at USD 8.76 Bn in 2025 and is expected to reach USD 19.73 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 12.3% from 2025 to 2032.
The companion diagnostics market plays a vital role in precision medicine by helping healthcare providers identify patients who are most likely to benefit from targeted therapies. It improves treatment effectiveness, enhances safety, and supports better clinical outcomes, especially in oncology, infectious diseases, and chronic conditions. Advances in molecular diagnostics, growing biomarker discovery, strong collaboration between pharmaceutical and diagnostic companies, and wider adoption of personalized healthcare across hospitals and laboratories worldwide continue to drive the market forward.
|
Current Events |
Description and its impact |
|
Regulatory and Policy Advancements |
|
|
Technological Innovations and Integration |
|
|
Pharmaceutical Industry Trends |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Artificial intelligence plays a critical role in companion diagnostics by enabling precise, data-driven identification of patients most likely to benefit from specific therapies. AI algorithms analyze complex biomedical data—such as digital pathology images, genomic profiles, and biomarker patterns—to improve diagnostic accuracy, consistency, and speed. In oncology, AI enhances tissue-based assays by detecting subtle features beyond human interpretation, supporting treatment selection and clinical trial stratification.
In September 2025, Lunit, a provider of AI solutions for cancer diagnostics and therapeutics, and Agilent Technologies Inc., a global life sciences and diagnostics company, announced a nonexclusive collaboration to develop AI-based companion diagnostics. The partnership combines Lunit’s AI capabilities with Agilent’s expertise in tissue-based companion diagnostic development to address the growing need for advanced biomarker assays in drug development.
Real Time-Polymerase Chain Reactions (PCR) hold the largest market share of 42.2% in 2025. Real-time polymerase chain reaction (PCR) strengthens the companion diagnostics market by enabling rapid and highly precise detection of genetic mutations and clinically relevant biomarkers used in therapy selection. It delivers quantitative results quickly, allowing clinicians to make timely treatment decisions, especially in oncology and infectious disease care. The growing adoption of targeted therapies, rising demand for dependable molecular testing in hospitals, ongoing assay advancements, and seamless integration of PCR systems into routine clinical workflows further reinforce its importance in companion diagnostics. For instance, in December 2025, Roche Diagnostics China launches its first locally developed quantitative polymerase chain reaction (PCR) system for the Chinese market.
Infectious diseases fuel growth in the companion diagnostics market as clinicians depend more on accurate tests to select targeted antiviral and antimicrobial treatments. Rapid identification of pathogens, resistance indicators, and disease variants increases the demand for advanced diagnostic technologies. Rising awareness of emerging infections, ongoing improvements in molecular testing, and the use of diagnostics in infectious disease drug development further support adoption. Hospitals and laboratories increasingly implement companion diagnostics to enable faster treatment decisions and enhance overall patient care.
Hospitals propel the companion diagnostics market by acting as key hubs for patient diagnosis, treatment planning, and therapy monitoring. Their broad and varied patient populations generate steady demand for biomarker-based tests, while equipped laboratories and skilled staff facilitate advanced molecular diagnostics. Hospitals actively implement personalized medicine programs, partner with pharmaceutical and diagnostic firms, and invest in innovative diagnostic technologies. By embedding companion diagnostics into clinical workflows, they improve treatment accuracy, enhance patient outcomes, and boost the use of targeted therapies across diverse medical areas. For instance, in December 2025, Guardant Health, Inc. launched FPG 360, its in-house liquid biopsy testing service, at Policlinico Gemelli in Rome, Italy.
To learn more about this report, Download Free Sample
North America dominates the overall market with an estimated share of 36.8% in 2025. Advancements in precision medicine and molecular diagnostics are shaping key trends in the North America companion diagnostics market. Healthcare providers increasingly use biomarker-based tests to select targeted therapies, especially in oncology and infectious disease care. Pharmaceutical companies and diagnostic developers actively collaborate to launch innovative assays, while hospitals and laboratories invest in advanced diagnostic technologies. Supportive regulatory frameworks and reimbursement policies further encourage adoption. Together, these trends enable personalized treatment strategies and integrate companion diagnostics more fully into routine clinical practice across the region. For instance, in May 2025, NeoGenomics, Inc., a leading oncology testing provider, launched c-MET CDx, its companion diagnostic IHC assay for NSCLC.
The Europe companion diagnostics market is advancing with a strong emphasis on personalized medicine and sophisticated molecular testing. Healthcare providers increasingly apply biomarker-based diagnostics to select targeted therapies in oncology, cardiovascular, and infectious disease care. Pharmaceutical companies, diagnostic developers, and research institutions actively collaborate to drive innovation and launch new assays. Hospitals and specialized laboratories invest in state-of-the-art technologies, while supportive regulations and rising awareness among clinicians and patients promote wider adoption, further integrating companion diagnostics into routine clinical practice across Europe. For instance, in November 2024, Roche received CE Mark for VENTANA® FOLR1 RxDx Assay, the first widely available IHC companion diagnostic in Europe to identify EOC patients eligible for ELAHERE® treatment.
The United States companion diagnostics market is growing quickly as healthcare providers increasingly adopt precision medicine and molecular diagnostic technologies. Clinicians actively use biomarker-based tests to select targeted therapies, especially in oncology and infectious disease care. Pharmaceutical and diagnostic companies collaborate to develop and launch innovative assays, while hospitals and clinical laboratories invest in advanced diagnostic platforms. Supportive regulatory frameworks and reimbursement policies encourage wider implementation, collectively promoting personalized treatment approaches and integrating companion diagnostics more fully into U.S. healthcare practice. For instance, in September 2025, the FDA approved Guardant360 CDx as a companion diagnostic to identify ESR1-mutated advanced breast cancer patients eligible for imlunestrant (Inluriyo).
The United Kingdom companion diagnostics market is expanding with a strong focus on personalized medicine and advanced molecular testing. Healthcare providers actively use biomarker-based diagnostics to select targeted therapies, especially in oncology and rare diseases. Pharmaceutical companies, diagnostic developers, and research institutions collaborate to develop and launch innovative assays. Hospitals and specialized laboratories invest in state-of-the-art technologies, while supportive healthcare policies and increasing clinician awareness drive adoption. Together, these trends promote patient-specific treatment strategies and integrate companion diagnostics into routine clinical practice across the UK. For instance, in March 2025, Abingdon Health extended its distribution agreement with Salignostics to launch an own-brand Salistick and leverages its CDMO division to take lateral flow projects from concept to large-scale manufacturing.
The companion diagnostics market is increasingly driven by the global shift toward precision and personalized medicine. Clinicians rely on biomarker-based tests to identify patients who will benefit most from targeted therapies, improving treatment efficacy and reducing adverse effects. This trend encourages pharmaceutical companies to co-develop drugs and companion diagnostics, while hospitals and laboratories invest in advanced molecular testing platforms, integrating diagnostics directly into clinical decision-making to support individualized patient care.
Pharmaceutical companies increasingly require companion diagnostics to support targeted therapies during clinical trials. Developing co-branded diagnostic assays presents opportunities for companies to partner with drug developers, enhancing patient stratification, trial efficiency, and regulatory compliance. Hospitals and research institutions benefit from early access to validated tests, enabling better monitoring of therapeutic responses. This integration strengthens the value proposition of companion diagnostics, creates revenue opportunities, and accelerates the approval and adoption of novel precision therapies.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 8.76 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 12.3% | 2032 Value Projection: | USD 19.73 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
F. Hoffmann-La Roche AG, Agilent Technologies, Inc., QIAGEN N.V, Abbott Laboratories, Inc., Almac Group, Danaher Corporation, Illumina, Inc., bioMérieux SA, Myriad Genetics, Inc., Sysmex Corporation, Thermo Fisher Scientific Inc., Abnova Corporation, Guardant Health, Inc., Icon Plc., and Biogenex Laboratories, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients