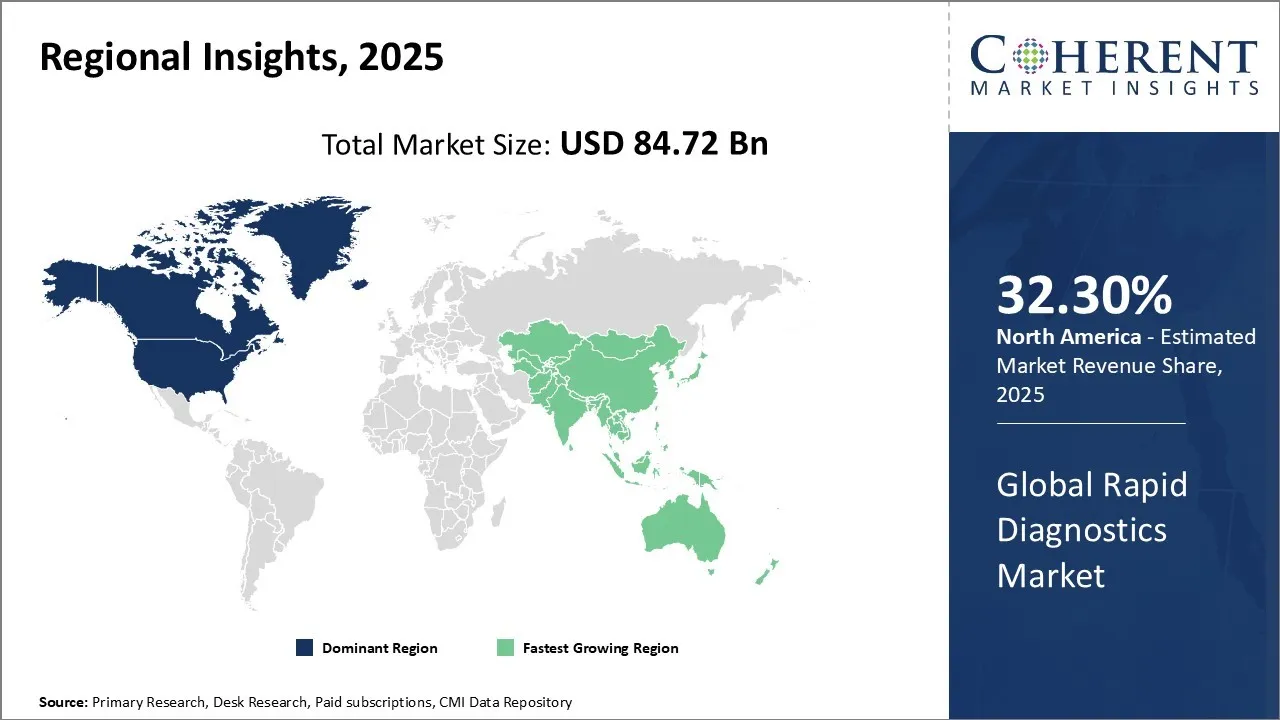
Rapid Diagnostics Market is estimated to be valued at USD 84.72 Bn in 2025 and is expected to reach USD 163.01 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 9.8% from 2025 to 2032.
The rapid diagnostics market is growing rapidly due to the rising need for quick, accurate, and accessible disease detection. These tests provide results within minutes, enabling faster clinical decisions and timely treatment. High prevalence of infectious and chronic diseases, along with increased demand for point-of-care and at-home testing, is driving rapid diagnostics market demand.
Advancements in technologies like lateral flow assays and biosensors are improving test performance. Government support for early diagnosis and the expansion of telehealth further boosts market growth. Rapid diagnostics are becoming essential for decentralized, patient-centric healthcare delivery.
|
Current Event |
Description and Its Impact |
|
Regulatory Policy Shifts |
|
|
Healthcare System Transformations |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
In terms of prescription, the professional diagnostics segment is projected to dominate the global rapid diagnostics market growth in 2025, due to the rising need for fast and accurate decision making. In hospitals and diagnostic labs, clinicians rely on professional-grade rapid diagnostic tools for quick turnaround times often within minutes to guide immediate treatment decisions. This is crucial in emergency care, infectious disease outbreaks, or intensive care units where delays can compromise outcomes. With the global rise in conditions like respiratory infections, sepsis, diabetes, and cardiovascular diseases, professional diagnostic tools are essential for high-volume, high-accuracy screening and monitoring in healthcare facilities. These products help detect diseases in early stages and prevent complications. Professional diagnostic products are designed to integrate seamlessly into laboratory and hospital information systems (LIS/HIS), supporting faster reporting, better compliance, and traceability. This level of integration is essential for regulated environments like hospitals and national screening programs.
In May 2025, MP Biomedicals, a global life‑sciences company, launched a new line of advanced diagnostic kits targeting gastrointestinal diseases. The kits offer enhanced sensitivity and specificity, catering to healthcare professionals seeking rapid and reliable results. Designed for clinical laboratories and diagnostic centers, these solutions streamline testing workflows while supporting accurate disease detection.
In terms of product, the consumables segment is expected to contribute the largest share in the market in 2025, due to their critical role in every test procedure and their high usage frequency. Most rapid diagnostic tests (RDTs) rely on disposable consumables test strips, cartridges, reagents, sample collection kits, pipettes, and swabs that are used once per test and then discarded. This drives continuous, repeat demand, especially in high-volume testing environments. As rapid diagnostics become more common in point-of-care, home-based, and decentralized healthcare settings, the number of tests conducted has grown significantly. More tests mean higher consumption of associated consumables, such as lateral flow assay components or buffer solutions. Consumables are needed across multiple applications from infectious disease testing (e.g., HIV, malaria) to chronic disease monitoring (e.g., glucose, cholesterol). Each diagnostic segment uses unique test kits and reagents, all of which are consumable-driven. For diagnostic companies, consumables offer a recurring revenue stream, unlike diagnostic instruments, which are typically one-time purchases. This incentivizes manufacturers to design systems that rely on proprietary or compatible consumables, ensuring ongoing demand.
In May 2024, Revital Healthcare, in partnership with USAID, launched a new rapid test kit production facility in Kilifi, Kenya. The facility can produce up to 20 million kits per month, covering diseases such as HIV, malaria, hepatitis B and C, dengue, syphilis, and HCG.
In terms of application, the blood glucose testing segment is expected to command the largest share of 60.7% of the market in 2025, due to rising prevalence of diabetes and real-time monitoring. Diabetes affects over 500 million people globally, and this number is steadily increasing, especially in low- and middle-income countries. Frequent monitoring of blood glucose is essential for diabetes management, driving demand for fast, accessible testing solutions. Blood glucose levels can fluctuate rapidly based on diet, activity, and medication. Rapid diagnostics allow immediate, point-of-care results, helping patients and providers make timely decisions on insulin or dietary adjustments. Portable glucometers and continuous glucose monitors (CGMs) have made it possible for patients to test at home. This aligns with the growing trend of decentralized healthcare and patient self-management, reducing the burden on clinics and labs.
In June 2025, IBM and Roche introduced the Accu‑Chek SmartGuide Predict app, an AI‑enabled solution paired with Roche’s continuous glucose monitoring (CGM) sensor. The app delivers rapid diagnosis-style alerts, forecasting glucose trends up to two hours ahead, warning of low blood sugar up to 30 minutes in advance, and providing overnight risk notifications.
In terms of end user, the hospitals and clinics segment is expected to command the largest share in 2025 due to their critical need for speed, accuracy, and operational efficiency in patient care. Hospitals and emergency departments require immediate diagnostic results to make timely treatment decisions, especially in acute or life-threatening conditions such as sepsis, stroke, or respiratory infections. Rapid diagnostics reduce wait times from hours (or days) to minutes, improving patient outcomes. With rising patient volumes, hospitals and clinics rely on streamlined testing workflows to reduce bottlenecks in laboratories and outpatient departments. Rapid diagnostic tools minimize dependency on centralized labs and enable quick turnaround, helping facilities manage caseloads more efficiently. Clinics use rapid tests for routine monitoring of chronic diseases such as diabetes (glucose), cardiovascular issues (cholesterol, troponin), and kidney conditions (creatinine), often during the same patient visit, which enhances convenience and compliance.
In May 2025, NHS Forth Valley launched a new Rapid Cancer Diagnostic Service (RCDS) aimed at accelerating cancer diagnoses for patients who have concerning symptoms but don’t meet the usual criteria for urgent cancer referrals.
To learn more about this report, Download Free Sample
North America is expected to gain highest share in the market over the forecast period owing to the increasing prevalence of chronic and infectious disease, growing demand for point of care (POC) diagnostics, and rise in adoption of portable rapid testing devices in the region. A notable example includes the ongoing use and expansion of Abbott’s ID NOW system across urgent care and pharmacy chains in the U.S., where rapid molecular tests for flu, and RSV are being offered with results in under 15 minutes. These devices have become especially popular during seasonal outbreaks, where rapid detection is critical for early intervention and containment.
Additionally, public health agencies have partnered with diagnostic companies to expand access in rural and underserved areas. For instance, mobile testing units equipped with rapid antigen and molecular platforms were deployed in 2023 to address respiratory illness surges in remote parts of Canada and the U.S. This strong regional momentum reflects growing rapid diagnostics market demand, as healthcare providers shift toward decentralized, patient-friendly solutions that deliver accurate results at the point of need.
Europe is experiencing robust growth in the rapid diagnostics market, driven by a surge in infectious and chronic diseases, wider adoption of point-of-care (POC) diagnostics, and strong government support. The European Centre for Disease Prevention and Control (ECDC) is now actively monitoring a rising wave of tropical infections including dengue, West Nile virus, and chikungunya in regions that were previously unaffected, as mosquito populations expand due to climate change; over 300 local dengue cases in 2024, and chikungunya appearing earlier in 2025, highlight the urgency. These developments have prompted public health authorities to scale up rapid testing capabilities to support early outbreak detection and control.
Additionally, Europe has launched an EU-wide wastewater surveillance dashboard to track respiratory pathogens such as SARS-CoV-2, influenza, and RSV in near real-time, significantly enhancing the continent’s ability to anticipate and respond to health threats. By integrating this data with rapid diagnostic testing, European health systems are adopting a more proactive and layered approach to disease surveillance.
These initiatives including expanded POC testing in clinics, laboratories, and pharmacies—underscore the growing Rapid Diagnostics Market share in Europe. Governments and health institutions are investing heavily in rapid detection tools that can deliver fast, accurate results outside traditional labs, reinforcing the market’s growth trajectory.
The United States is a key driver of the rapid diagnostics market, supported by a strong healthcare system, high chronic disease burden, and widespread use of point-of-care (POC) testing. In July 2025, PHASE Scientific, a biotech company focused on science-driven healthcare innovation, announced an exclusive U.S. distribution agreement with Lumos Diagnostics for FebriDx®—a rapid point-of-care test that uses a single drop of blood to distinguish bacterial from non-bacterial acute respiratory infections in about 10 minutes. The demand for at-home diagnostic kits is rising, especially for conditions like flu, and diabetes.
The FDA has fast-tracked several OTC rapid tests, including combined flu kits, to boost accessibility. Hospitals and clinics increasingly use rapid cardiac and glucose diagnostics for quicker decision-making. This focus on early detection and convenience continues to fuel strong rapid diagnostics market in the U.S.
Germany is a key driver in the rapid diagnostics market due to its strong diagnostic industry and government-backed initiatives for early disease detection.
Germany’s successful pharmacy-based virus and flu testing model has expanded to include rapid tests for flu, RSV, and strep. This approach improves access to diagnostics while easing the burden on hospitals. The rising concerns over antimicrobial resistance have also spurred demand for fast, targeted testing boosting rapid diagnostics market across the country.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 84.72 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.8% | 2032 Value Projection: | USD 163.01 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Abbott, Danaher, Quidel, BD, LifeScan, Siemens Healthineers, F. Hoffmann-La Roche, and Ascensia Diabetes Care Holdings, among others. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
One of the key factors expected to augment the global rapid diagnostics market growth over the forecast period is the rise in prevalence of infectious/chronic diseases worldwide. For instance, with the increasing prevalence of infectious diseases, the use/adoption of rapid diagnostics devices is also increasing with the rapid pace. In January 2023, Cipla announced the launch of a diagnostic device to test various medical conditions, such as infectious diseases, cardiac markers, diabetes, thyroid function, and others. Cippoint will allow healthcare professionals to get test results in 3 to 15 minutes, enabling a faster clinical decision-making process. This is further accelerating the rapid diagnostics market demand.
Government support is playing a critical role in accelerating the growth of the rapid diagnostics market, particularly through funding, regulatory fast-tracking, and public health programs aimed at improving early disease detection and accessibility to testing. Many national healthcare strategies now prioritize point-of-care and decentralized diagnostics as essential components of public health infrastructure.
In both developed and developing countries, governments are investing in rapid diagnostic tools to strengthen pandemic preparedness, reduce diagnostic turnaround times, and support large-scale screening programs. For example, several nations have introduced subsidies and procurement schemes to distribute rapid test kits for malaria, tuberculosis, and sexually transmitted infections. These actions have significantly boosted both production and public adoption of rapid diagnostics.
Additionally, regulatory bodies like the FDA, EMA, and CDSCO have implemented emergency use authorizations (EUAs) and accelerated approval pathways for rapid diagnostic technologies encouraging innovation and faster market entry. Initiatives like India’s National Health Mission, the U.S. Biomedical Advanced Research and Development Authority (BARDA) funding, and the EU’s Horizon programs have further contributed to research, development, and local manufacturing of diagnostic tools.
According to rapid diagnostics market research, such policy support is expected to continue driving innovation, affordability, and deployment of rapid diagnostic solutions, particularly in rural healthcare, infectious disease control, and chronic disease management.
With the increasing number of diagnostic laboratories and rapid technological advancements, the use and/or demand for rapid diagnostics tests is also increasing rapidly, worldwide. For instance, according to National Accreditation Board for Testing and Calibration Laboratories (NABL) as of September 2021, there are around 8000 NABL accredited medical labs present in India. This trend is expected to continue over the forecast period, driving the market growth.
The rapid diagnostics market value is undergoing a fundamental shift—from a supplementary diagnostic approach to a front-line enabler of healthcare accessibility, precision medicine, and real-time decision-making.
A clear inflection point can be seen in the uptake of rapid molecular diagnostics in antimicrobial resistance (AMR) control. The European Centre for Disease Prevention and Control (ECDC) noted a 38% increase in the deployment of rapid AMR detection assays across hospitals in Germany and the Netherlands between 2020 and 2024. This surge is a direct response to physician demand for immediate, actionable microbial profiles—an area where traditional diagnostics are increasingly seen as inadequate. In my opinion, the growing prioritization of test-to-treat workflows by healthcare payers further legitimizes this shift.
Moreover, the integration of digital health with rapid diagnostics is another underappreciated catalyst. A telling example comes from the Indian subcontinent, where private labs and public health programs are using app-integrated lateral flow tests to screen for dengue and leptospirosis in rural districts. These low-resource settings are generating some of the world’s highest diagnostic yields per dollar spent, redefining efficiency benchmarks in global health.
Walgreens and CVS Health in the United States now stock over 20 rapid tests for conditions ranging from influenza to sexually transmitted infections. These aren’t gimmicks—they are reflections of a deeper consumerization of healthcare. However, my contention is that regulatory lag and uneven data interoperability are constraining the potential of this shift. Until rapid test data is routinely integrated into EHRs and national surveillance systems, its full value remains unrealized.
Additionally, there is an emerging discrepancy in quality standards versus innovation velocity. Many startups, particularly in Asia-Pacific, are outpacing traditional manufacturers in test development cycles but lack harmonized validation frameworks.
*Definition: Rapid diagnostic tests (RDTs), also known as rapid tests, are easy-to-use tests that provide quick results, usually in minutes or less. These are quick field tests that can be performed in under 30 minutes and detect the presence of a pathogen quickly and accurately.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients