Uterine Fibroid Treatment Devices Market Size and Forecast – 2025 – 2032
The Global Uterine Fibroid Treatment Devices Market size is estimated to be valued at USD 1.15 billion in 2025 and is expected to reach USD 2.14 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 9.3% from 2025 to 2032.
Global Uterine Fibroid Treatment Devices Market Overview
Uterine fibroid treatment devices encompass a variety of minimally invasive and noninvasive systems developed to manage fibroid growth and related symptoms. These include radiofrequency ablation devices, focused ultrasound systems, embolization catheters, and surgical tools for laparoscopic or hysteroscopic myomectomy. The latest innovations emphasize tissue selectivity, shorter recovery times, and fertility preservation. Advanced imaging-guided systems enable precise targeting, while robotic-assisted platforms enhance surgical control and visualization. The trend toward outpatient and uterus-sparing procedures continues to shape the evolution of these devices.
Key Takeaways
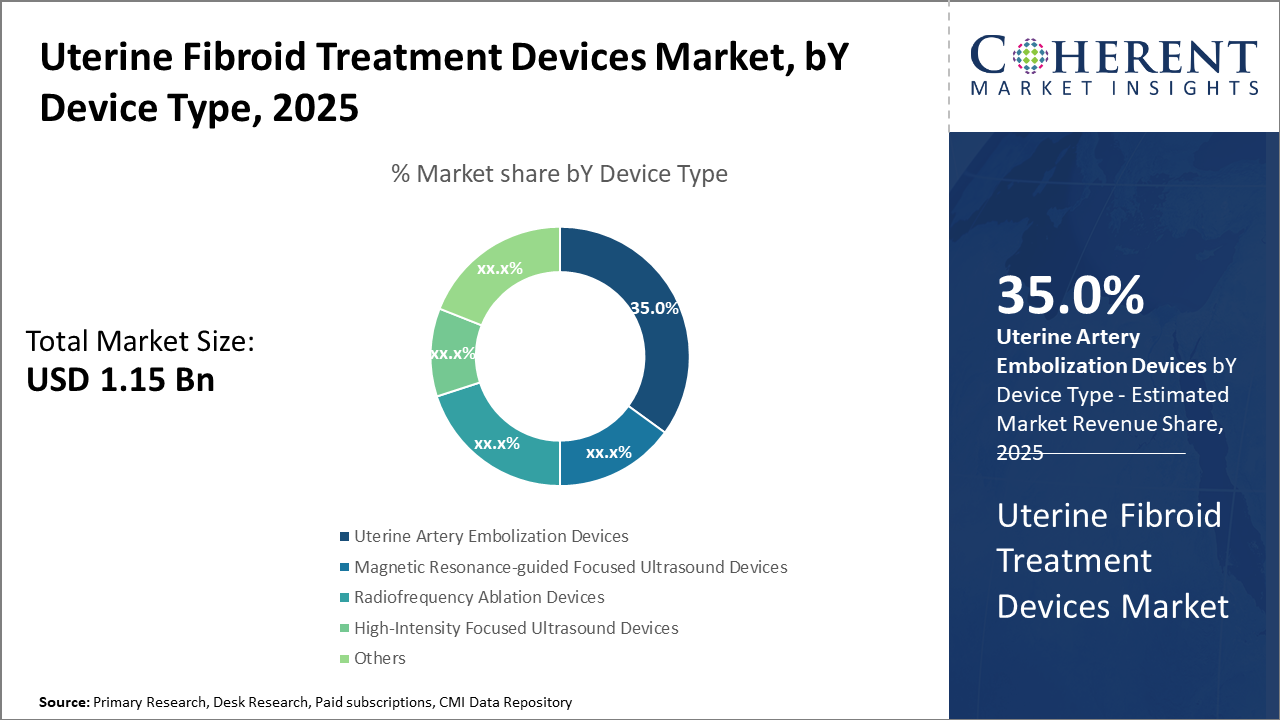
The uterine artery embolization device segment leads with a 35% market share, driven by efficacy and minimal invasiveness.
Hospitals remain the dominant end-user segment due to access to sophisticated treatment infrastructure and specialist expertise.
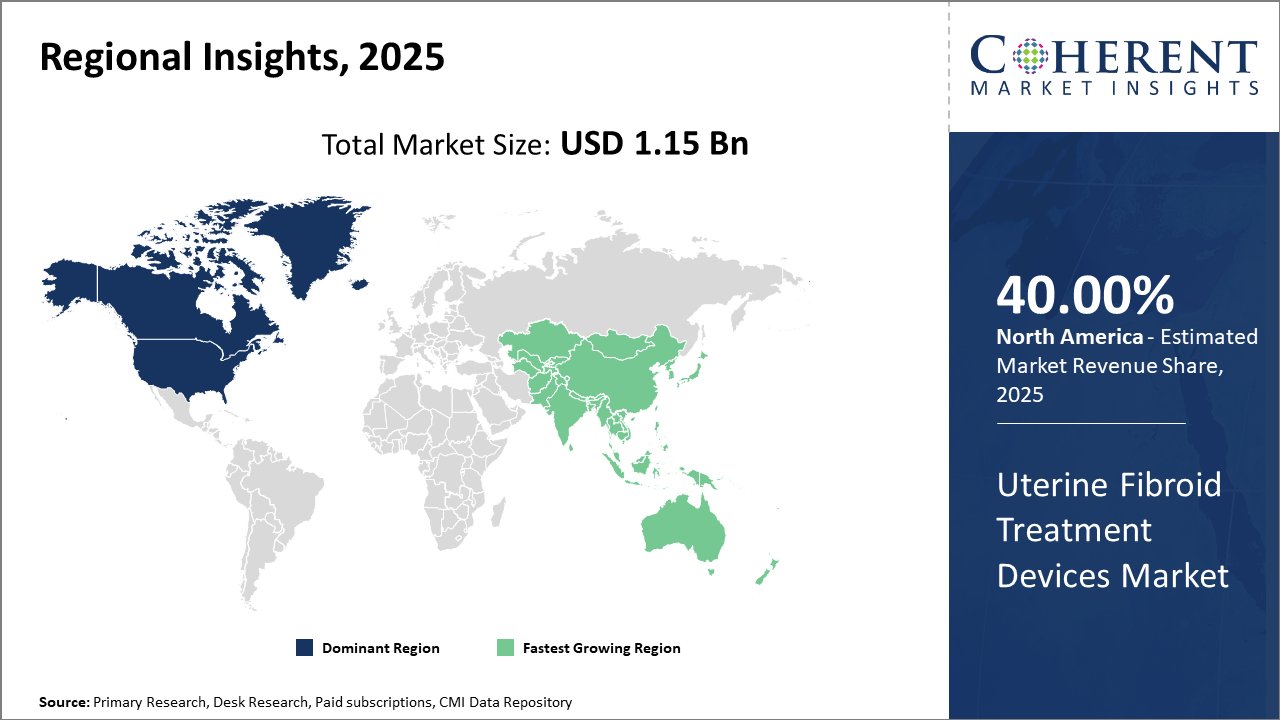
North America maintains industry share leadership, accounting for approximately 40% of market revenue, supported by strong healthcare infrastructure and reimbursement policies.
Asia Pacific showcases the highest CAGR, propelled by expanding healthcare access, increasing awareness, and localized manufacturing initiatives in countries such as China and India.
Uterine Fibroid Treatment Devices Market Segmentation Analysis
To learn more about this report, Download Free Sample
Uterine Fibroid Treatment Devices Market Insights, By Device Type
In terms of device type, the Uterine Artery Embolization Devices dominate the market share at 35%. This dominance is attributed to their proven clinical efficacy in minimizing invasiveness while preserving the uterus's integrity, creating high demand, especially in developed healthcare systems. Meanwhile, magnetic resonance-guided focused ultrasound devices are the fastest-growing subsegment, benefiting from technological advancements that offer non-invasive, incisionless treatment options attractive to a broader patient base. Radiofrequency ablation and high-intensity focused ultrasound devices also maintain stable demand as key complementary treatments, with smaller shares accounting for device customization needs.
Uterine Fibroid Treatment Devices Market Insights, By End-User
Hospitals dominate due to their capacity to support complex procedures and an integrated treatment infrastructure. Hospitals continue to lead due to their ability to provide multidisciplinary care, critical in managing uterine fibroids and associated comorbidities. Specialty clinics represent a growing subsegment, especially in urban centers where outpatient management of uterine fibroid treatment devices is gaining traction. Ambulatory surgical centers, while smaller in market share, are expanding rapidly, facilitated by advancements in device portability and efficacy, thereby appealing to cost-sensitive patients seeking quicker recovery times.
Uterine Fibroid Treatment Devices Market Insights, By Application
Symptomatic Uterine Fibroids dominate the market share, given the larger patient pool requiring intervention. This segment drives the largest market revenue since the majority of uterine fibroid patients present with symptoms severe enough to warrant device-based treatment. The infertility-related cases subsegment is the fastest growing, driven by increased research highlighting device safety in preserving fertility, prompting gynecologists and patients to consider advanced uterine fibroid treatment devices earlier.
Uterine Fibroid Treatment Devices Market Trends
Recent market trends reveal substantial adoption of minimally invasive uterine fibroid treatment devices, with magnetic resonance-guided focused ultrasound gaining preference due to zero-incision approaches.
In 2024, regulatory approvals for new generation radiofrequency ablation devices marked a milestone, improving patient outcomes and driving market share.
Additionally, integration of AI with imaging technology is improving treatment precision, while digital health platforms enhance postoperative care, setting new standards for the uterine fibroid treatment devices market.
Uterine Fibroid Treatment Devices Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Uterine Fibroid Treatment Devices Market Analysis and Trends
In North America, the dominance in the uterine fibroid treatment devices market is attributed to robust healthcare infrastructure, higher healthcare expenditure, and favorable reimbursement policies. The U.S., especially, shoulders approximately 28% of global market revenue, driven by extensive clinical adoption of uterine artery embolization and radiofrequency ablation devices. Innovation-centric companies headquartered in the region, such as Boston Scientific Corporation and Medtronic, continuously introduce advanced product lines, fueling growth in this market. Strong patient awareness and prevalence of fibroid cases propel sustained industry share in this region.
Asia Pacific Uterine Fibroid Treatment Devices Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth with a CAGR exceeding 11% through 2032. This growth is fueled by increasing healthcare access, rising prevalence of uterine fibroids, and accelerated adoption of minimally invasive uterine fibroid treatment devices. Countries like China and India lead expansion due to increased government funding for women's health programs and a surge in private healthcare investment. Market companies are increasingly localizing production to optimize device pricing and broaden market penetration.
Uterine Fibroid Treatment Devices Market Outlook for Key Countries
USA Uterine Fibroid Treatment Devices Market Analysis and Trends
The USA’s uterine fibroid treatment devices market benefits from high healthcare expenditure and early adoption of innovative technologies. Boston Scientific and Medtronic dominate the market with comprehensive portfolios in uterine artery embolization and radiofrequency ablation devices. Clinical trials and FDA approvals in 2024 for advanced focused ultrasound systems significantly increased the market revenue by 12% compared to the previous year. Surgeons’ preference for uterus-conserving treatments and insurance coverage expansion ensure the USA remains a pivotal contributor to global market size and revenue.
India Uterine Fibroid Treatment Devices Market Analysis and Trends
India’s market experiences rapid growth, supported by rising awareness about fibroid treatment options and expanding hospital infrastructure in both urban and semi-urban areas. Local production initiatives have reduced device costs by nearly 10% in recent years, improving access to uterine fibroid treatment devices. Leading market companies such as Hologic and Siemens Healthineers are actively entering partnerships with Indian healthcare providers to drive adoption, contributing to impressive market growth trajectories. Government efforts to improve women’s healthcare schemes are further bolstering business growth within the country.
Analyst Opinion
The demand-side dynamics in the uterine fibroid treatment devices market show an increasing preference for minimally invasive procedures. For instance, the rate of adoption for uterine artery embolization devices grew by over 22% in 2024, reflecting shifting patient and clinician inclination towards less invasive treatment. This trend is a strong contributor to the expanding market size and market revenue year over year.
On the supply side, production capacity enhancements and technological innovation have significantly optimized device efficacy and reduced associated post-procedural complications. In 2025, manufacturers introduced next-generation radiofrequency ablation devices with 30% improved energy delivery efficiency, leading to shortened procedure times and lower hospital stays. This has driven a competitive edge within market players aiming to scale manufacturing rapidly.
Market analysis reveals expanding application use cases across various patient demographics, particularly among women aged 30 to 50 who prioritize fertility preservation. The increasing number of clinical trials studying device impact on reproductive outcomes significantly drives market growth strategies, indicating a favorable market forecast for uterine fibroid treatment devices. Reports from 2024 indicate a 15% rise in demand linked to better patient compliance with device-supported procedures.
Pricing dynamics also play a critical role, as emerging markets witness increased accessibility to therapy options. For example, selective markets in Asia-Pacific have seen average device pricing decline by 8% in 2024 due to improved supply chain efficiencies and local manufacturing, fueling higher overall market share. These factors collectively influence the global industry size and market scope.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 1.15 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.1% | 2032 Value Projection: | USD 2.14 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Boston Scientific Corporation, Hologic, Inc., Siemens Healthineers, Medtronic PLC, Cook Medical LLC, GE Healthcare, EDAP TMS S.A., Philips Healthcare, Terumo Corporation, MiraCare, Inc., Cynosure, Inc. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Uterine Fibroid Treatment Devices Market Growth Factors
The increasing prevalence of symptomatic uterine fibroids globally is the foremost driver pushing demand for advanced treatment devices, notably as fibroids affect nearly 70% of women by age 50 in several countries. Secondly, technological innovation in minimally invasive therapies continues to alter market dynamics by improving efficacy and reducing recovery times, driving enhanced adoption in clinical settings. Third, rising healthcare expenditure and improved insurance coverage in developed economies enable greater patient access to specialized devices, supporting sustained market growth. Finally, ongoing clinical awareness programs and growing patient preference for uterus-sparing treatment options are critical to enhancing market scope and contributing to healthy CAGR progression.
Uterine Fibroid Treatment Devices Market Development
In May 2024, Meditrina, Inc. received U.S. FDA 510(k) clearance for its Gen 2 Bipolar RF Hysteroscopy System and the accompanying Aveta Glo device. The system is designed for minimally-invasive intrauterine procedures and features bipolar radio-frequency (RF) energy for more controlled resection and coagulation of pathology. The new Aveta Glo disposable RF device expands procedural versatility by enabling treatment of fibroids and calcified intra-uterine tissue with deeper myometrial penetration and integrated hemostasis, expected to improve operating-room efficiency and outcomes.
In May 2025, Hologic, Inc. received CE-Mark approval for its new MyoSure Synergy System, a single-use, handheld hysteroscopic tissue-removal device intended for the efficient removal of fibroids and other intra-uterine tissue. The launch supports the company’s strategy to deliver office- and ambulatory-based solutions for women’s health by simplifying workflows, reducing set-up and enabling broader adoption of tissue-removal procedures.
Key Players
Leading Companies of the Market
Boston Scientific Corporation
Hologic, Inc.
Siemens Healthineers
Medtronic PLC
Cook Medical LLC
GE Healthcare
EDAP TMS S.A.
Philips Healthcare
Terumo Corporation
MiraCare, Inc.
Cynosure, Inc.
Several leading companies have adopted expansion and innovation-driven strategies to maintain competitive advantage. For example, Boston Scientific enhanced its market revenue by extending its uterine artery embolization device portfolio, capturing a significant share in North America and Europe. Meanwhile, Hologic implemented strategic partnerships to accelerate R&D in high-intensity focused ultrasound devices, enabling faster clinical adoption and positioning it strongly in Asia Pacific markets. These approaches contribute directly to market growth.
Uterine Fibroid Treatment Devices Market Future Outlook
In the coming years, uterine fibroid treatment devices will continue advancing toward outpatient and noninvasive modalities. Real-time image-guided platforms, robotic-assisted systems, and AI-driven procedural mapping will enhance precision. Disposable device components will improve sterility and reduce maintenance costs. Integration with digital health platforms for post-procedure monitoring and patient engagement is expected to become standard. As awareness of minimally invasive gynecologic care increases, these devices will be positioned as first-line treatment options, replacing many traditional surgeries.
Uterine Fibroid Treatment Devices Market Historical Analysis
Treatment for uterine fibroids historically involved invasive surgical interventions such as hysterectomy and myomectomy. The 2000s brought a major transition toward less invasive options, with the introduction of uterine artery embolization and focused ultrasound ablation systems. Energy-based technologies, such as radiofrequency and cryoablation, provided tissue-selective alternatives that preserved uterine integrity. Continuous refinement in catheter and imaging technologies improved procedural safety and efficacy. This evolution was driven by a growing preference for fertility preservation, faster recovery, and lower complication rates.
Sources
Primary Research Interviews:
Gynecologic Surgeons
Interventional Radiologists
Biomedical Device Engineers,
Reproductive Health Specialists
Databases:
FDA Device Approvals
GlobalData Medical Devices Database
WHO Reproductive Health Data
Magazines:
MedTech Insight
Diagnostic and Interventional Cardiology
Medical Design & Outsourcing
OBG Management
Journals:
American Journal of Obstetrics & Gynecology
Journal of Minimally Invasive Gynecology
Ultrasound in Obstetrics & Gynecology
The Lancet Women’s Health
Newspapers:
The Guardian (Health)
The New York Times (Science)
The Times of India (Wellness)
Bloomberg Health
Associations:
American College of Obstetricians and Gynecologists (ACOG)
Society of Interventional Radiology (SIR)
World Health Organization (WHO)
European Society for Gynecological Endoscopy (ESGE)
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients