U.S. Urea Cycle Disorders Treatment Market is estimated to be valued at USD 547.0 Mn in 2025 and is expected to reach USD 698.8 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 3.56% from 2025 to 2032. Increasing product approvals for urea cycle disorders and increasing number of pipeline products is expected to boost the growth of the market over the forecast period. Moreover, increasing adoption of inorganic growth strategies by key players for Urea Cycle Disorders Treatment by regulatory bodies is expected to propel the growth of the U.S. Urea Cycle Disorders Treatment market over the forecast period
Analysts’ Views on U.S. Urea Cycle Disorders Treatment market:
Manufacturers are focusing on increasing the number of pipeline products for urea cycle disorders and the development of novel treatments for urea cycle disorder, which is expected to propel the market growth of the U.S. urea cycle disorder treatment market over the forecast period.
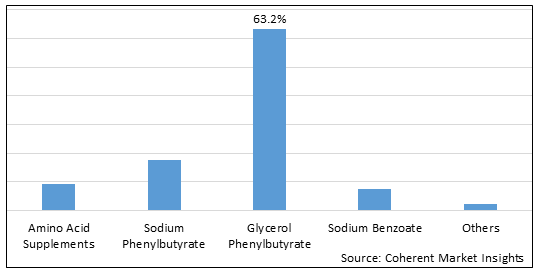
Figure 1. U.S. Urea Cycle Disorders Treatment Market Share (%), by Treatment Type, 2025
To learn more about this report, Download Free Sample
U.S. Urea Cycle Disorders Treatment Market - Driver
Increasing product approvals for urea cycle disorders
Increasing product approvals for urea cycle disorders treatment by regulatory bodies is expected to drive market growth over the forecast period. For instance, in December 2022, Acer Therapeutics Inc., a pharmaceutical company, and its collaboration partner, Relief Therapeutics Holding AG, a commercial-stage biopharmaceutical company, announced that the U.S. Food and Drug Administration (FDA) had approved OLPRUVA (sodium phenylbutyrate) for oral suspension in the U.S. for the treatment of certain patients living with urea cycle disorders (UCDs) involving deficiencies of carbamylphosphate synthetase (CPS), ornithine transcarbamylase (OTC), or argininosuccinic acid synthetase (AS).
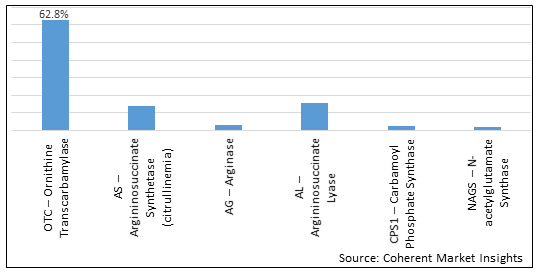
Figure 2. U.S. Urea Cycle Disorders Treatment Market Share (%), by Enzyme Deficiency Type, 2025
To learn more about this report, Download Free Sample
U.S. Urea Cycle Disorders Treatment Market– Impact of Coronavirus (COVID-19) Pandemic
Supply chain and manufacturing activities in the U.S. have been disrupted due to lockdowns implemented by governments in the past year, and also facing problems with regards to the transportation of drug products. The coronavirus or COVID-19 outbreak that started in Wuhan, China has spread across continents, affecting various industries globally. The supply of key materials has been severely disrupted due to forced quarantine, and a lack of labor and raw materials. As the link between regional warehouses is not smooth, the transportation of raw materials between regions cannot be carried out successfully. This shortage of raw materials and components has affected the supply chain of the U.S. urea cycle disorders treatment market. Due to the severe shortage of medical resources at the front line, only patients diagnosed with serious conditions can be hospitalized. Unfortunately, the pathogenic mechanism of the virus has not been identified completely, therefore, there is no specific drug and treatment except for symptomatic and supportive treatments. Respiratory support devices such as life-support machines, atomizers, oxygen generators, and monitors are primary clinical treatment medical devices. Thus, from diagnosis to cure, the need for instruments for measuring temperature, nucleic acid diagnostic kits, antiviral medical products, and life-support machines has increased consistently. Furthermore, the COVID-19 pandemic has impacted the global as well as the U.S. economy and in turn, the urea cycle disorders treatment market.
U.S. Urea Cycle Disorders Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 547.0 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 3.56% | 2032 Value Projection: | USD 698.8 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Horizon Therapeutics Plc, Bausch Health Companies Inc, Recordati Rare Diseases Inc., Nestlé S.A., Danone S.A., Lucane Pharma SA, Acer Therapeutics Inc., Ultragenyx Pharmaceutical Inc., Aeglea Biotherapeutics, Inc., Arcturus Therapeutics Holdings Inc., Orpharma Pty Ltd., Selecta Biosciences, Inc., Abbott, and Mead Johnson & Company, LLC |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
U.S. Urea Cycle Disorders Treatment Market Segmentation:
U.S. Urea Cycle Disorders Treatment market is segmented into Treatment Type, Enzyme Deficiency Type, Route of Administration, and Distribution Channel
Based on Treatment Type, the market is segmented into Amino Acid Supplements, Sodium Phenylbutyrate, Glycerol Phenylbutyrate, Sodium Benzoate, and Others. Due to key players focusing on strategies such as product acquisition to strengthen their product portfolio for the treatment of UCDs, the Glycerol Phenylbutyrate segment accounted for a significant share of 62.8% in the U.S. market in 2025.
In terms of Enzyme Deficiency Type, the U.S. Urea Cycle Disorders Treatment market has been segmented into OTC – Ornithine Transcarbamylase, AS – Argininosuccinate Synthetase (citrullinemia), AG – Arginase, AL – Argininosuccinate Lyase, CPS1 – Carbamoyl Phosphate Synthase, and NAGS – N-acetylglutamate Synthase. Due to increasing incidence of the N-acetylglutamate synthase deficiency in the U.S., N-acetylglutamate Synthase (NAGS) segment is expected to dominate the market over the forecast period.
On the basis of Route of Administration, the market has been divided into Oral and Injectable. Due to the Increasing approvals of oral suspension by regulatory bodies, the Oral segment is expected to dominate the market over the forecast period.
On the basis of Distribution Channel, the market has been divided into Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies. Due to major players are focusing on running special awareness programs for the treatment of urea cycle disorders through healthcare facilities, the Hospital pharmacies segment expected to dominate the market over the forecast period.
U.S. Urea Cycle Disorders Treatment Market: Key Developments
Increasing collaboration for urea cycle disorders treatment by market players is expected to propel the growth of the U.S. Urea Cycle Disorders Treatment market over the forecast period. For instance, in January 2021, Recordati Rare Diseases Inc., a biopharmaceutical company, received the U.S. Food and Drug Administration (FDA) approval for CARBAGLU for the treatment of acute hyperammonemia due to propionic acidemia (PA) or methylmalonic acidemia (MMA) in pediatric. CARBAGLU was previously approved by U.S. Food Drug and Administration in the year 2021 for the treatment of acute hyperammonemia due to N-acetylglutamate synthase (NAGS) deficiency.
U.S. Urea Cycle Disorders Treatment Market: Restraint
High Cost Of Urea Cycle Disorders Treatment
Drugs such as RAVICTI is a nitrogen-binding agents approved for the treatment of chronic management of urea cycle disorder (UCD) in adults and children. RAVICTI is developed by Horizon Therapeutics plc. a global biotechnology company, and it costs around US$ 793,632 for a year of treatment of a patient suffering from urea cycle disorder. RAVICTI is one of the most expensive drugs available in the U.S. for the treatment of UCD.
U.S. Urea Cycle Disorders Treatment Market - Key Players
Major players operating in the U.S. Urea Cycle Disorders Treatment market include Horizon Therapeutics Plc, Bausch Health Companies Inc, Recordati Rare Diseases Inc., Nestlé S.A., Danone S.A., Lucane Pharma SA, Acer Therapeutics Inc., Ultragenyx Pharmaceutical Inc., Aeglea Biotherapeutics, Inc., Arcturus Therapeutics Holdings Inc., Orpharma Pty Ltd., Selecta Biosciences, Inc., Abbott and Mead Johnson & Company, LLC
Definition: Urea cycle disorders (UCDs) are a group of orphan inherited defects of six enzymes and two transporters that constitute the urea cycle in the periportal liver cells that affect how the body removes the waste that is made from the breaking down of protein. The symptoms of urea cycle disorders vary in severity and result from the excessive accumulation of ammonia in the blood and body tissues called hyperammonemia. Common symptoms include lack of appetite, vomiting, drowsiness, seizures, and/or coma. In severe cases, if the urea cycle disorder the liver may be abnormally enlarged, which is called hepatomegaly
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients