Ornithine Transcarbamylase (OTC) Deficiency Treatment Market is estimated to be valued at USD 880.3 Mn in 2025 and is expected to reach USD 1,178.0 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 4.25% from 2025 to 2032. Increasing research and development activities for Ornithine Transcarbamylase (OTC) deficiency treatment by market players is expected to boost growth of the market over the forecast period. Moreover, increasing product approvals for Ornithine Transcarbamylase (OTC) deficiency treatment by regulatory bodies is expected to propel growth of the global Ornithine Transcarbamylase (OTC) deficiency treatment market over the forecast period
Analysts’ Views on Global Ornithine Transcarbamylase (OTC) deficiency treatment market:
Urea cycle disorders (UCD) caused by OTC deficiency, can be potentially fatal for patients amidst of the COVID-19 pandemic, as companies in the Ornithine Transcarbamylase (OTC) deficiency treatment market are ramping up their production to increase the availability of IV and oral drugs. For long-term management of ammonia in blood streams, gene therapy and nitrogen scavenger therapy are gaining popularity. Since, there are no U.S. FDA-approved drugs for OTC deficiency, companies in the Ornithine Transcarbamylase (OTC) deficiency treatment market should strengthen their R&D capabilities in order to innovate in IV medications. Key companies should innovate in the field of dietary supplements because daily protein and supplement intake will help to improve patient’s quality of life.
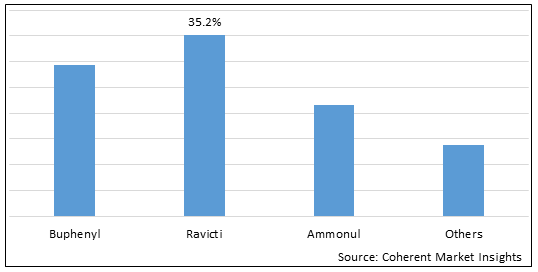
Figure 1. Global Ornithine Transcarbamylase (OTC) Deficiency Treatment Market Share (%), by Drug Type, 2025
To learn more about this report, Download Free Sample
Global Ornithine Transcarbamylase (OTC) Deficiency Treatment Market - Driver
Increase in the research and development activities by market players
Increasing research and development activities for Ornithine Transcarbamylase (OTC) deficiency treatment by market players is expected to drive the market growth over the forecast period. For instance, in April 2020, Arcturus Therapeutics, a clinical-stage messenger RNA medicine company, announced the acceptance of two clinical trials for its flagship asset ARCT-810, also known as LUNAR-OTC, a first-in-class mRNA therapeutic that is being developed to treat Ornithine Transcarbamylase (OTC) deficiency. The company’s Investigational New Drug (IND) application for Phase 1b study in patients with OTC deficiency was allowed to proceed by the U.S. Food and Drug Administration (FDA), and an additional Clinical Trial Application (CTA) for a Phase 1 study in healthy volunteers was approved by the New Zealand Medicines and Medical Devices Safety Authority (Medsafe).
Ornithine Transcarbamylase (OTC) Deficiency Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 880.3 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 4.25% | 2032 Value Projection: | USD 1,178.0 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Horizon Therapeutics Plc, Bausch Health Companies Inc., Danone, Nestlé, Ultragenyx Pharmaceutical., Arcturus Therapeutics, Inc. , Abbott., Swedish Orphan Biovitrum AB, Acer Therapeutics Inc., Assertio Holdings, Inc., iECURE, and Translate Bio, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
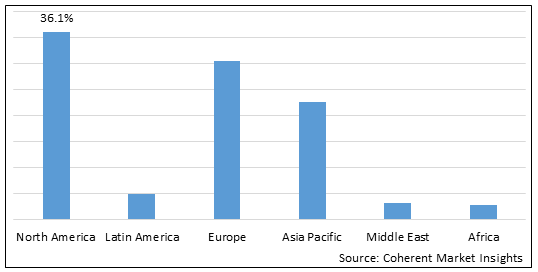
Figure 2. Global Ornithine Transcarbamylase (OTC) Deficiency Treatment Market Share (%), by Region, 2025
To learn more about this report, Download Free Sample
Global Ornithine Transcarbamylase (OTC) Deficiency Treatment Market- Regional Analysis
Among regions, North America is expected to dominate the market over the forecast period, owing to North America’s 36.1% market share in 2025 and increasing product approvals for the treatment of Ornithine Transcarbamylase (OTC) deficiency, which is expected to drive the global Ornithine Transcarbamylase (OTC) deficiency treatment market growth in the region. For instance, in August 2022, iECURE, a gene editing company focused on developing therapies that utilizes mutation-agnostic in- vivo gene insertion, or knock-in, editing for the treatment of liver disorders with significant unmet need, announced that the U.S. Food and Drug Administration (FDA) had granted Rare Pediatric Disease Designation to its lead product candidate, GTP-506, for the treatment of Ornithine Transcarbamylase (OTC) deficiency, a rare genetic condition that can lead to irreversible neurological impairment, seizures, coma, and death, in pediatric population.
Europe is expected to be second largest region in global Ornithine Transcarbamylase (OTC) deficiency treatment market over the forecast period due to increasing research and development activities by key players in the region. For instance, in October 2021, Boehringer Ingelheim, a research-driven biopharmaceutical company, and Thoeris GmbH, a developer of therapeutic interventions intended for treating orphan diseases, announced a collaboration and license agreement with the aim to investigate novel first-in-class therapies for patients with urea cycle disorders (UCDs). UCDs are rare diseases caused by genetic liver dysfunctions, leading to excess ammonia levels in the blood and there is insufficient treatment available for this disease till date.
Global Ornithine Transcarbamylase (OTC) Deficiency Treatment Market– Impact of Coronavirus (COVID-19) Pandemic
The supply of raw materials required for production of pharmaceuticals has been severely disrupted due to the forced quarantine and lack of labor during the COVID-19 pandemic. As the link between regional warehouses was not smooth, transportation of raw materials between regions was not carried out successfully. This shortage of raw materials and components negatively affected the supply chain of the global Ornithine Transcarbamylase (OTC) deficiency treatment market. Thus, COVID-19 pandemic is expected to have negative impact on supply chain of global Ornithine Transcarbamylase (OTC) deficiency treatment market.
According to an article published by the Exploratory Research in Clinical and Social Pharmacy journal, in June 2021, a study was carried out to evaluate the impact of COVID-19 on pharmaceutical systems and supply chain in resource-limited countries in Sub-Saharan countries, such as Namibia. This study revealed a negative impact on availability and access of essential drugs, sanitation, hygiene products, and antimicrobials. Most pharmaceutical companies and pharmacies in Namibia experienced delayed manufacturing and distribution of drugs, and this attributed to reduced inter-country transportation of pharmaceutical goods and limited in-country capacity to manufacture drugs.
For instance, according to a review article published by the European Pharmaceutical Review journal, in November 2020, COVID-19 pandemic has caused major challenges with respect to drug shortages and increased manufacturing costs across the globe. Moreover, the same source stated that COVID-19 pandemic has also led to problems such as stockpiling drugs, transportation delay, and others. Some measures that can be taken to ease the supply of pharmaceuticals during the pandemic included next generation technologies such as digital network platforms designed to work across multiple pharmaceutical enterprises and to ensure the timely delivery of drugs to patients across the globe.
Global Ornithine Transcarbamylase (OTC) Deficiency Treatment Market Segmentation:
Global Ornithine Transcarbamylase (OTC) deficiency treatment market is segmented into Drug Type, Route of Administration, and Distribution Channel
Based on Drug Type, the market is segmented Buphenyl, Ravicti, Ammonul, and Others. Due to increased efficacy, drug cost, and improved patient compliance, Ravicti segment accounted for a significant share 35.2% in the global market in 2025.
In terms of Route of Administration, the global Ornithine Transcarbamylase (OTC) deficiency treatment market has been segmented into oral and intravenous. Due to patient preference for oral route and the convenience it provides, approvals and launches of oral medication, availability of a large number of products that can be administered through the oral route, and new products with improved formulation modification for taste masking, the oral segment is expected to dominate the market over the forecast period.
On the basis of Distribution Channel, market has been divided into hospital pharmacies, retail pharmacies, and online pharmacies. Due to the higher number of prescriptions filled at thehospital pharmacies, favorable reimbursement scenario, strong supply chain management, and various patient assistance programme run by pharmaceutical companies, the hospital pharmacies segment expected to dominate the market over forecast period.
Global Ornithine Transcarbamylase (OTC) deficiency treatment market- Cross Sectional Analysis:
Among drug type, Ravicti segment expected to exhibit highest CAGR, in Asia Pacific region due to the key players in the market focusing on agreements for Ravicti in Asia Pacific. For instance, in May 2022, Immedica Pharma AB, a private European niche pharma group, and OrphanPacific, Inc., a Japan-based pharmaceutical company, announced that the companies have entered into an agreement, under which OrphanPacific gained the exclusive rights to Ravicti in Japan. Under the announced agreement, OrphanPacific is granted a license to develop, register, and commercialize the product in urea cycle disorders in Japan.
Among distribution channel, hospitals pharmacies segment is dominant in North America region due to growing hospital expenditure by governments, which is expected to provide strong growth prospects to the market during the forecast period. For instance, according to the U.S. Centers for Medicare and Medicaid Services 2021 findings on the National Health Expenditure (NHE) in the U.S., the hospital expenditure grew by 6.4% to reach US$ 1,270.1 billion in 2020, as compared to hospital expenditure in 2019. Additionally, prescription drug spending increased by 3.0% to reach US$ 348.4 billion in 2020, as compared to 2019.
Global Ornithine Transcarbamylase (OTC) Deficiency Treatment Market: Key Developments
Increasing number of initiatives for research and development activities for Ornithine Transcarbamylase (OTC) deficiency treatment market by market players is expected to propel the growth of the Global Ornithine Transcarbamylase (OTC) deficiency treatment market over the forecast period. For instance, in April 2021, Ultragenyx Pharmaceutical Inc., a biopharmaceutical company focused on the development and commercialization of novel therapies for rare and ultra-rare diseases, announced the successful completion of End-of-Phase 2 (EOP2) meeting with the U.S. Food and Drug Administration (FDA), for the DTX301 Ornithine Transcarbamylase (OTC) deficiency gene therapy program. The meeting focused on the discussion of the Phase 1/2 data and alignment on Phase 3 design and endpoints. Based on the outcome of this meeting, Ultragenyx has finalized the Phase 3 study design, which will include a 64-week primary efficacy analysis period and enroll approximately 50 patients 12 years of age and older, randomized 1:1 to DTX301 (1.7 x 10^13 GC/kg dose) or placebo.
Global Ornithine Transcarbamylase (OTC) Deficiency Treatment Market: Restraint
High cost of Ornithine Transcarbamylase (OTC) Deficiency Treatment
Drugs such as RAVICTI are a nitrogen-binding agent that are approved for the treatment of chronic management of urea cycle disorder (UCD) in adults and children. RAVICTI is developed by Horizon Therapeutics and it costs around US$ 793,632 for a year of treatment of patient suffering from urea cycle disorder. RAVICTI is one of the most expensive drugs available in the U.S. for the treatment of Ornithine Transcarbamylase (OTC) deficiency. Government should focus on reimbursement policies for Ornithine Transcarbamylase (OTC) deficiency to boost the growth of the market.
Global Ornithine Transcarbamylase (OTC) Deficiency Treatment Market - Key Players
Major players operating in the global Ornithine Transcarbamylase (OTC) deficiency treatment market include Horizon Therapeutics Plc, Bausch Health Companies Inc, Danone, Nestlé, Ultragenyx Pharmaceutical., Arcturus Therapeutics, Inc., Abbott., Swedish Orphan Biovitrum AB, Acer Therapeutics Inc., Assertio Holdings, Inc., iECURE, and Translate Bio, Inc.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients