Shigella Vaccines Market Size and Forecast – 2025 – 2032
The Shigella Vaccines Market size is estimated to be valued at USD 1.85 billion in 2025 and is expected to reach USD 3.75 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 10.5% from 2025 to 2032.
Global Shigella Vaccines Market Overview
Shigella is a gram-negative pathogenic enterobacteria that causes severe diarrhea and dysentery in humans. Symptoms associated with Shigella infection includes fever, stomach pain, tenesmus, watery diarrhea, vomiting, dehydration, and convulsions. Various strains of Shigella are encompassed, such as S. dysenteriae, S. flexneri, S. sonnei and S. boydii. Each species of Shigella has different serotypes classified on the basis of the structure of O-antigens repeats that are the polysaccharides moiety of the lipopolysaccharide, a virulence factor consist of toxic lipid.
According to World Health Organization (WHO), nearly one million people die from Shigella infection annually. Moreover, the high incidence rate is seen among children less than five years age, travelers, and military personnel from industrialized economies. Shigella species have been identified from centuries and it represents a major threat to public health, due to non-availability of licensed vaccine and thus affecting overall Shigella vaccines market.
Key Takeaways
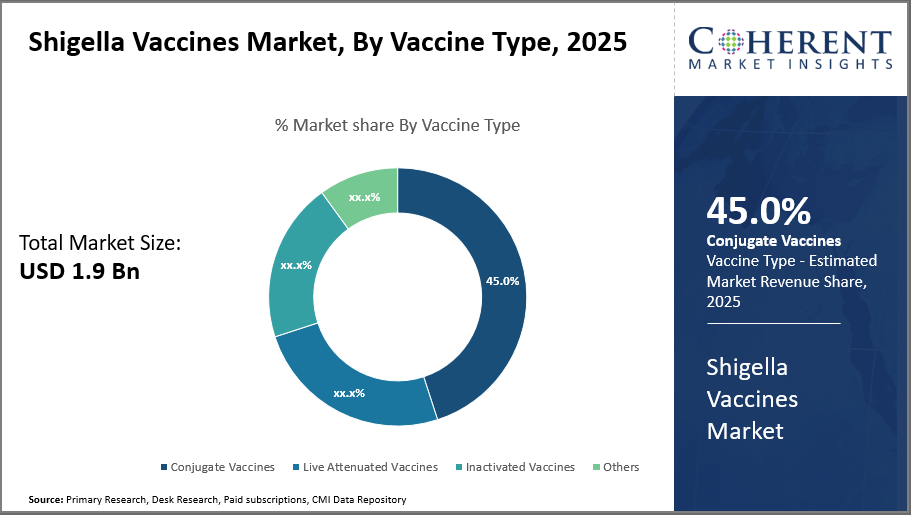
The Conjugate Vaccines segment retains dominance with 45% industry share due to its established efficacy and widespread adoption globally.
Government Clinics constitute the largest Distribution Channel segment, accounting for 40% market revenue, driven by concentrated immunization campaigns.
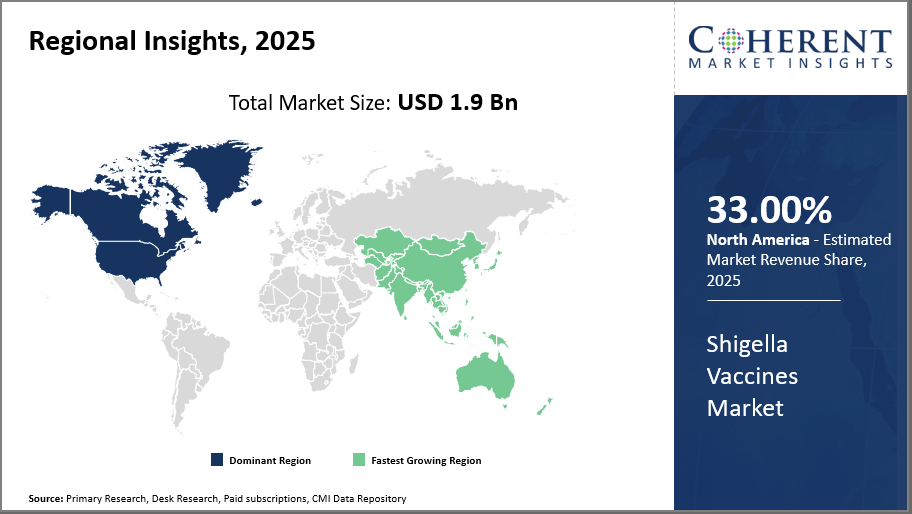
Regionally, North America leads the Shigella Vaccines Market with a commanding market share of 34%, benefiting from advanced healthcare infrastructure and significant R&D investments. Asia Pacific emerges as the fastest-growing region, exhibiting a CAGR of 16.8%, propelled by expanding healthcare access and supportive government policies in India and China.
Shigella Vaccines Market – Segmentation Analysis
To learn more about this report, Download Free Sample
Shigella Vaccines Market Insights, By Vaccine Type
In terms of Vaccine Type, Conjugate Vaccines dominate the market share with 45%. This dominance is attributed to their proven high efficacy and the ability to confer protection against multiple Shigella serotypes, making them the preferred choice in government immunization programs. These vaccines offer advantages such as better immunogenicity and the potential for inclusion in combination vaccine formulations with other enteric pathogens. Experts recognize that conjugate vaccines may improve vaccination acceptance, reduce logistical complexities, and diminish administration errors compared to standalone vaccines.
Shigella Vaccines Market Insights, By End User
Pediatric Immunization Programs dominate the market share with 35%, driven by increasing government initiatives prioritizing childhood immunization against diarrheal diseases in high-burden regions. Further, the World Health Organization (WHO) prioritizes accelerating the development and accessibility of safe, effective, and affordable Shigella vaccines for this age group to reduce deaths and long-term health consequences.
Shigella Vaccines Market Insights, By Distribution Channel
In terms of Distribution Channel, Government Clinics leading the market at 40% share. This predominance is due to widespread public health immunization delivery systems in both developed and developing countries. Government clinics are the main locations for immunizing vulnerable groups, including children under five, who are a major focus of Shigella immunization campaigns. With the help of national health ministries and WHO programs, public health officials utilize government clinics to make vaccines widely available and reasonably priced.
Shigella Vaccines Market Trends
The Shigella Vaccines market is witnessing a significant paradigm shift towards mRNA-based vaccine technologies, which promise rapid adaptability against evolving Shigella strains. Moderna and BioNTech have initiated preclinical evaluations of mRNA candidates with encouraging immunogenic data in 2024.
Another emerging trend is the rising integration of digital immunization tracking and AI-driven cold chain monitoring systems across vaccine distribution channels, enhancing vaccine efficacy and reducing wastage. Countries like the U.S. and Germany reported up to 30% improvement in vaccine delivery efficiency after implementing such technologies in 2025.
Apart from this, the growing involvement of philanthropic organizations in financing Shigella vaccine deployment in low-income regions is reshaping market dynamics, offering expanded access and creating new demand pockets. The Gates Foundation’s grants in East Africa in 2024 enabled vaccination coverage to reach an estimated additional 3 million children.
Shigella Vaccines Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Shigella Vaccines Market Analysis and Trends
In North America, the dominance in the Shigella Vaccines market stems from well-established biotechnology ecosystems, strong governmental funding for vaccine R&D, and widespread healthcare infrastructure. The presence of major companies like Pfizer and Moderna contributes to the market share. Regulatory streamlining and advanced vaccine distribution networks further solidify this region's market leadership.
Asia Pacific Shigella Vaccines Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth with an anticipated CAGR exceeding 12%. Growth is supported by increasing disease burden in densely populated countries such as India and China. Rising government focus on public vaccination programs and expanding local vaccine manufacturing capacities, exemplified by Serum Institute of India's scale-up in 2024, underpin rapid market expansion.
Shigella Vaccines Market Outlook for Key Countries
United States Shigella Vaccines Market Analysis and Trends
The U.S. market is propelled by heavy investment in vaccine innovation and public immunization infrastructure. With key market players headquartered here, such as Pfizer and Moderna, the U.S. boasts advanced R&D pipelines focusing on mRNA and conjugate formulations. Recent CDC immunization campaigns targeting high-risk populations have increased vaccine uptake by 18% in 2025. Furthermore, favorable regulatory pathways have expedited vaccine approvals, facilitating early commercial launches and influencing overall market revenue in the country.
China Shigella Vaccines Market Analysis and Trends
The growing incidence of Shigella infections and the growing need for efficient preventative measures are expected to propel the market for Shigella vaccines in China to substantial expansion. The market is growing as a result of China's developing healthcare system, benevolent regulatory framework, and government programs to combat infectious diseases. To address unmet medical requirements, major players in the vaccine industry are aggressively investing in research and development to bring cutting-edge Shigella vaccines to market.
Analyst Opinion
Enhanced Production Capacities and Technological Advancements — Recent expansions in vaccine production infrastructures, especially in biotech hubs across North America and Asia Pacific, have significantly increased global output. In 2024, new manufacturing facilities employing cutting-edge cell-culture technologies contributed to a 12% uplift in production capacity compared to 2023, directly influencing market size and supply reliability.
Demand Surge Driven by End-User Diversification — Increasing adoption of Shigella vaccines in pediatric immunization programs and humanitarian efforts in endemic regions has widened demand. For instance, immunization campaigns in India’s high-incidence districts in 2025 reported a 20% annual growth in vaccination coverage, further catalyzing market revenue expansion.
Pricing Dynamics and Government Subsidies — Strategic pricing adjustments supported by government subsidies in Latin America and Africa have lowered vaccination costs, stimulating market uptake. The introduction of tiered pricing models in Brazil in late 2024 resulted in a 15% rise in public health procurement, highlighting pricing as a pivotal market driver.
Export Patterns and Regulatory Harmonization — Enhanced export volume from manufacturing-heavy countries combined with streamlined regulatory approvals in Europe and Asia has paved the way for smoother market penetration. Reports indicate a 25% year-over-year increase in export volumes from India and South Korea in early 2025, underpinning significant market growth from supply-side dynamics.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 1.85 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 10.5% | 2032 Value Projection: | USD 3.75 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Serum Institute of India, Pfizer Inc., GlaxoSmithKline plc, Bharat Biotech International Ltd, Janssen Pharmaceuticals (Johnson & Johnson), Sanofi Pasteur, Moderna Inc., Novavax Inc., Takeda Pharmaceutical Company, Sinovac Biotech | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Shigella Vaccines Market Growth factors
The surge in shigellosis prevalence and corresponding need for effective vaccines is a primary market growth driver. Recent epidemiological data showed a 9% rise in reported Shigella cases in sub-Saharan Africa during 2024, intensifying urgency for vaccination solutions.
Government backing and increased funding towards immunization campaigns have played a pivotal role, particularly in emerging economies such as India and Brazil, where vaccine inclusion in national immunization programs rose by 15% in 2025.
Technological breakthroughs in conjugate vaccine formulations provide enhanced immunogenicity and broadened protection across multiple serotypes of Shigella, fostering market revenue growth. This was validated by Phase III clinical trial successes of novel candidates reported in 2024.
Shigella Vaccines Market Development
In June 2025, GSK plc, announced that it has licensed its Shigella vaccine candidate, altSonflex1-2-3, to Bharat Biotech International Limited (BBIL). The agreement paves the way for the ongoing development and potential distribution of the vaccine in low-and-middle--income countries where Shigella, the leading bacterial cause of diarrhoea, poses a significant health threat to children under five.
In April 2025, Valneva SE, a specialty vaccine company, and LimmaTech Biologics AG, a clinical-stage biotech company developing vaccines for the prevention of life-threatening diseases, announced that the first participant has been vaccinated in a Phase 2 infant safety and immunogenicity study of Shigella4V2 (S4V2), the world’s most clinically advanced tetravalent bioconjugate vaccine candidate against shigellosis.
Key Players
Serum Institute of India
Pfizer Inc.
GlaxoSmithKline plc
Bharat Biotech International Ltd
Janssen Pharmaceuticals (Johnson & Johnson)
Sanofi Pasteur
Moderna Inc.
Novavax Inc.
Takeda Pharmaceutical Company
Sinovac Biotech
Co-development partnerships and cooperative license arrangements have become more popular among market participants as a means of accelerating pipeline developments. As an illustration, Pfizer's recent strategic equity agreement with BioNTech has improved the research capacity of mRNA-based vaccines, leading to quicker clinical trial success and increased market share in the United States. These partnerships have resulted in top corporations expanding their geographic reach and diversifying their product offerings.
Shigella Vaccines Market Future Outlook
The Shigella vaccines market will see significant progress driven by upcoming joint ventures and collaborations among major players who will focus on developing and commercializing effective vaccines. Research and clinical trials will advance, with vaccine candidates targeting multiple Shigella serotypes and combination vaccines that address multiple gastrointestinal pathogens showing promise. The development of thermostable vaccines will enhance accessibility in remote areas by reducing dependency on cold storage. There will be a growing emphasis on vaccines that will not only prevent symptomatic infection but also reduce long-term health impacts such as stunting and antimicrobial resistance.
Combination vaccines containing Shigella antigens along with other vaccines will gain higher acceptance due to benefits like simplified immunization schedules and reduced logistical burdens. Efforts will continue to harmonize clinical trial protocols and regulatory pathways to expedite safe and effective vaccine availability, particularly in low- and middle-income countries where the disease burden will be highest. Overall, the market outlook will suggest robust innovation and increasing prioritization of Shigella vaccines as crucial tools for global public health.
Historical Development
According to the Centers for Disease Control and Prevention (CDC), in 2013, the average annual incidence of shigellosis in the U.S. was around 5 cases per 100,000 individuals. Bacillary dysentery with brutal epidemics was caused by S. dysenteriae by the production of shiga toxins whereas, the endemic form of disease was caused by the S. flexneri and S. sonnei. The bacillary dysentery was accompanied with fever, rectal inflammation, and abdominal cramps.
For instance, in October 2019, The European Vaccine Initiative (EVI) and Hilleman Laboratories, announced partnership for the development of new shigella vaccine. Moreover, the EVI received a grant of US$ 9.5 Mn from the European and Developing Countries Clinical Trials Partnership (EDCTP) which aimed at developing safe and effective shigella vaccine.
Also, in March 2017, Hilleman Laboratories, a non-profit organization established with joint venture partnership between Merck & Co., and Wellcome Trust, entered into an agreement with National Institute of Cholera and Enteric Disease (NICED) for the development and commercialization of vaccines against Shigella by 2024. Besides, in June 2016, Immuron—an Australian biopharmaceutical company—entered into an agreement to produce Shigella vaccine with the U.S. Army’s biomedical research lab—Walter Reed Army Institute of Research (WRAIR).
Prokarium and Probiomed in January 2017, collaborated to manufacture orally-administrated vaccine to prevent diarrhea. The new vaccine was expected to be manufactured as a part of two-year collaboration having thermostable capabilities for a longer period. The new vaccine was expected to be delivered to the people living in the remote areas. Thermostable vaccine, which was manufactured at approximately one third of the price of the conventional injectable vaccines, helped to facilitate the growth of the Shigella vaccines market.
Sources
Primary Research interviews:
Public health officials (CDC, WHO, UNICEF representatives)
Clinical researchers working on enteric diseases
Vaccine manufacturers’ R&D heads
Databases:
Global Health Observatory (WHO)
UNICEF Data Portal
World Bank Health Data
Magazines:
BioPharm International
FiercePharma
PharmaTimes
Journals:
Journal of Infectious Diseases
Emerging Infectious Diseases
Nature Reviews Immunology
Newspapers:
The Washington Post (Science Section)
The Hindu (Health)
The Times of India (Health)
Associations:
Centers for Disease Control and Prevention (CDC)
Gavi, The Vaccine Alliance
PATH (Program for Appropriate Technology in Health)
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients