A conjugate vaccine is a type of subunit vaccine which combines a weak antigen with a strong antigen as a carrier so that the immune system has a stronger response to the weak antigen. Vaccines are used to prevent diseases by invoking an immune response to an antigen, part of a bacterium or virus that the immune system recognizes. This is usually accomplished with an attenuated or dead version of a pathogenic bacterium or virus in the vaccine, so that the immune system can recognize the antigen later in life. Most vaccines contain a single antigen that the body will recognize. However, the antigen of some pathogens does not elicit a strong response from the immune system, so a vaccination against this weak antigen would not protect the person later in life. In this case, a conjugate vaccine is used in order to invoke an immune system response against the weak antigen. In a conjugate vaccine, the weak antigen is covalently attached to a strong antigen, thereby eliciting a stronger immunological response to the weak antigen. Most commonly, the weak antigen is a polysaccharide that is attached to strong protein antigen. However, peptide/protein and protein/protein conjugates have also been developed.
Global conjugate vaccine market is estimated to be valued at US$ 18,012.08 million in 2022 and is expected to exhibit a CAGR of 9.6% during the forecast period (2022-2030).
Figure 1. Global Conjugate Vaccine Market Share, (%), Analysis, By Product Type, 2022
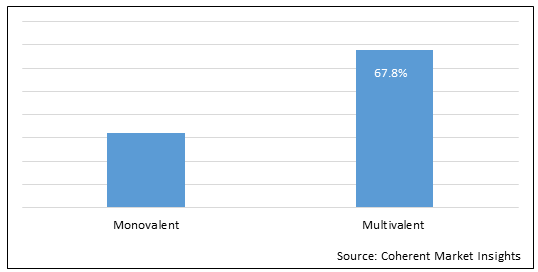
To learn more about this report, Download Free Sample
Increasing inorganic strategies such as partnerships between key market players are expected to drive growth of the global conjugate vaccine market over the forecast period.
Increasing inorganic strategies such as partnerships between key market players are expected to drive growth of the global conjugate vaccine market over the forecast period. For instance, in June 2021, VAXELIS (Diphtheria and Tetanus Toxoids and Acellular Pertussis, Inactivated Poliovirus, Haemophilus b Conjugate and Hepatitis B Vaccine), was developed as part of a U.S.-based partnership between Merck, a pharmaceutical company and Sanofi Pasteur, a pharmaceutical company, and was made available in the U.S. VAXELIS is the hexavalent (six-in-one) combination vaccine which is available in the U.S. and is indicated for active immunization to help prevent diphtheria, tetanus, pertussis, poliomyelitis, hepatitis B, and invasive disease due to Haemophilus influenzae type b. VAXELIS was approved by US FDA (Food and Drug Administration) for use as a 3-dose series in children 6 weeks through 4 years of age.
Conjugate Vaccine Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 18,012.08 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 9.6% | 2030 Value Projection: | US$ 37,587.25 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Sanofi S.A, Pfizer, Inc., Merck & Co., Inc., GlaxoSmithKline plc., Bharat Biotech, Serum institute of India Pvt. Ltd., Biological E. Limited, Bio-Med, Bavarian Nordic, CSL Limited, Novartis AG, Vaxcyte, GSBPL, Taj Pharmaceuticals Limited, and Bavarian Nordic. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Figure 2. Global Conjugate Vaccine Market Value (US$ Mn), By Region, 2021
To learn more about this report, Download Free Sample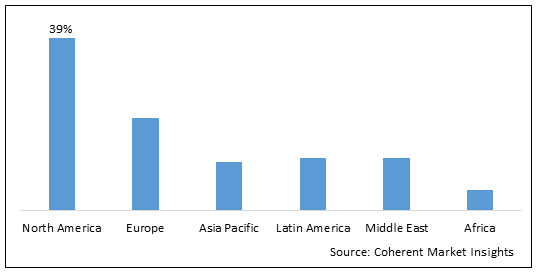
Increasing conjugate vaccine approval from the U.S. FDA (Food and Drug Administration) to key market players is expected to drive growth of the global conjugate vaccine market over the forecast period.
Increasing approval from the U.S. FDA (Food and Drug Administration) for conjugate vaccine to key market players is expected to drive growth of the global conjugate vaccine market over the forecast period. For instance, in July 2021, GlaxoSmithKline plc, a pharmaceutical company, announced that the U.S. Food and Drug Administration (FDA) had approved Shingrix (Zoster Vaccine Recombinant, Adjuvanted) for the prevention of shingles in adults aged 18 years and older who were at increased risk of shingles due to immunodeficiency or immunosuppression caused by known disease or therapy. Immunocompromised individuals were at greater risk of shingles and associated complications than immunocompetent individuals. Shingrix is a non-live, recombinant sub-unit adjuvanted vaccine, given intramuscularly in two doses.
Global Conjugate Vaccine Market – Impact of Coronavirus (COVID-19) Pandemic
The COVID-19 pandemic and lockdown in various countries across the globe has impacted the financial status of businesses across all sectors. The private healthcare sector is one such sector, which has been majorly impacted by the pandemic. COVID-19 has also affected the economies in three main ways- by directly affecting the production and demand, by creating disruptions in distribution channels, and through its financial impact on companies and financial markets.
Increasing vaccine approval from the U.S. FDA (Food and Drug Administration) during the pandemic has boosted growth of the global conjugate vaccine market. For instance, in August 2021, Pfizer Inc., a pharmaceutical company and BioNTech SE, a biotechnology company, had announced that the U.S. Food and Drug Administration (FDA) had approved the Biologics License Application (BLA) for COMIRNATY (COVID-19 Vaccine, mRNA) to prevent COVID-19 in individuals 16 years of age and older. The approval was based on a comprehensive submission package including six-month efficacy and safety data after second dose. Whereas, the vaccine has been available in the U.S. under Emergency Use Authorization (EUA) since December 11, 2020 (as the Pfizer-BioNTech COVID-19 Vaccine).Thus, impact of the Coronavirus (COVID-19) pandemic is expected to boost growth of the global conjugate vaccine market during the pandemic.
Global Conjugate Vaccine Market: Key Developments
Increasing collaborations among key players to develop and introduce vaccines for pneumococcal and meningococcal diseases in market and ongoing clinical trial studies on pneumococcal vaccines are expected to drive growth of the global conjugated vaccine market. For instance, in April 2018, Merck & Co, Inc., a pharmaceutical company, had announced that the company had begun Phase 3 studies of PCV-15 (V114), its investigational polyvalent conjugate vaccine for the prevention of pneumococcal disease. The first study) had evaluated the safety, tolerability and immunogenicity of PCV-15 followed by Pneumococcal Vaccine Polyvalent one year later in healthy adult subjects 50 years of age or older. The second Phase 3 study had evaluated the safety, tolerability and immunogenicity of PCV-15 followed by Pneumococcal Vaccine Polyvalent administered eight weeks later in adults infected with human immunodeficiency virus (HIV).
Increasing approval from the U.S. FDA (Food and Drug Administration) for meningococcal vaccine is expected to drive growth of the global conjugate vaccine market over the forecast period. For instance, in April 2020, The U.S. Food and Drug Administration (FDA) had approved a Biologics License Application for MenQuadfi Meningococcal Conjugate Vaccine for the prevention of invasive meningococcal disease in persons 2 years of age and older. MenQuadfi is the quadrivalent meningococcal vaccine in the U.S. that uses tetanus toxoid as a protein carrier. It is available in a ready-to-use liquid formulation allowing healthcare providers to avoid vaccine reconstitution.
Global Conjugate Vaccine Market – Restraints
Increasing number of side effects associated with various vaccines such as pneumococcal vaccine is expected to restrain growth of the global conjugate vaccine market over the forecast period. For instance, in February 2019, NHS, United Kingdom National Health Service, published a report on Pneumococcal vaccine side effects which stated that mild side effects of the pneumococcal conjugate vaccine (PCV), which is the version of the pneumococcal vaccine given to babies under the age of 2, include:
Key Players
Key players operating in the global conjugate vaccine market include Sanofi S.A, Pfizer, Inc., Merck & Co., Inc., GlaxoSmithKline plc., Bharat Biotech, Serum institute of India Pvt. Ltd., Biological E. Limited, Bio-Med, Bavarian Nordic, CSL Limited, Novartis AG, Vaxcyte, GSBPL, Taj Pharmaceuticals Limited, and Bavarian Nordic.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients