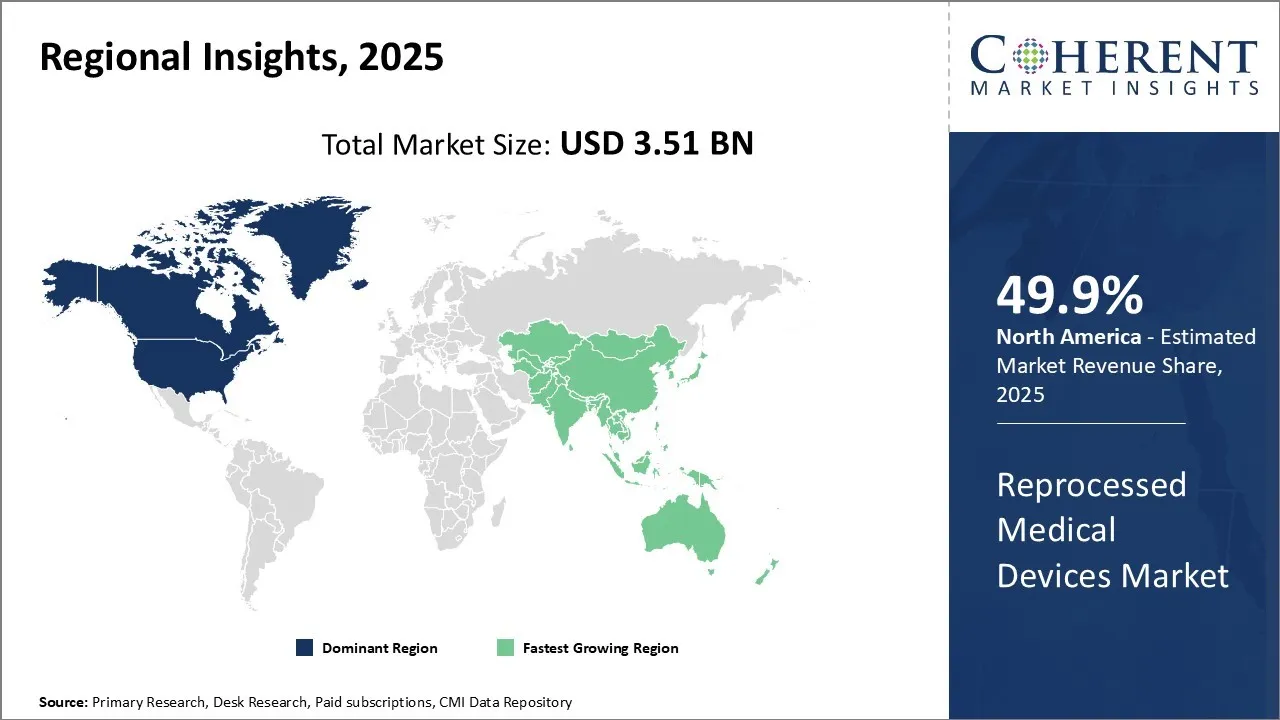
The reprocessed medical devices market is estimated to be valued at USD 3.51 Bn in 2025 and is expected to reach USD 9.57 Bn by 2032, growing at a compound annual growth rate (CAGR) of 15.4% from 2025 to 2032.
To learn more about this report, Download Free Sample
The reprocessed medical devices market is driven by the rising demand to lower healthcare costs. As medical devices are expensive, reprocessing helps reduce costs for patients and providers. The trend of improved reprocessing technologies is also supporting the market growth. Advancements in inspection and reconditioning techniques ensure that reprocessed devices meet high standards of safety and performance. Additionally, as the population ages and the burden of chronic diseases grows, more procedures will utilize these cost-efficient reprocessed alternatives to new medical devices. The market opportunity is substantial given the large replacement market for devices that are retrieved after use.
|
Current Events |
Description and its Impact |
|
Global Supply Chain Disruptions |
|
|
Growing Economic and Budgetary Pressures on Healthcare Providers |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
A reprocessed medical device that meets the FDA’s regulatory requirements is eligible for payment and coverage by Medicare on the same terms as a non-reprocessed device. Generally, Medicare reimburses devices that the FDA has legally approved under the Federal Food, Drug, and Cosmetic Act (FDCA), provided they are considered “reasonable and necessary” for diagnosing or treating an illness or injury, or for improving the function of a malformed body part.
Specifically, according to guidance from the Centers for Medicare & Medicaid Services (CMS), reprocessed devices that comply with FDA standards qualify for coverage under Medicare Part B just as non-reprocessed devices do. This same coverage approach extends to procedures performed with reprocessed devices in other Medicare-covered settings.
The device type segment includes laparoscopic devices, general surgery devices, orthopedic devices, cardiovascular devices, non-invasive devices, and others. The cardiovascular devices segment is anticipated to hold 37.3% of the market share in 2025. Cardiovascular diseases, particularly heart disease and stroke, are the leading causes of death globally. The rising sedentary lifestyles and unhealthy dietary patterns have led to a substantial increase in risk factors like obesity, diabetes and high blood pressure. This in turn is fueling the incidence of various heart conditions that require surgical procedures and devices for treatment. The aging population also presents a major driver as cardiovascular problems are highly common among the elderly. Moreover, technological advancements have enabled minimally invasive procedures using reprocessed cardiovascular devices, helping reduce medical costs significantly. The reuse of such devices provides sustainable solutions while ensuring safety and efficacy standards are met. Several governments and healthcare organizations worldwide are promoting reprocessing as a means to expand access to cardiovascular treatments, which is further reprocessed medical device market share.
In March 2025, MicroPort MedBot launched its Toumai SP laparoscopic surgical robot that have received approval from China’s National medical products Administration (NMPA). This launched is a significant advancement in the integration of robotic technology within China’s surgical landscape. The Toumai SP system is designed to enhance precision and control in minimally invasion surgeries. This
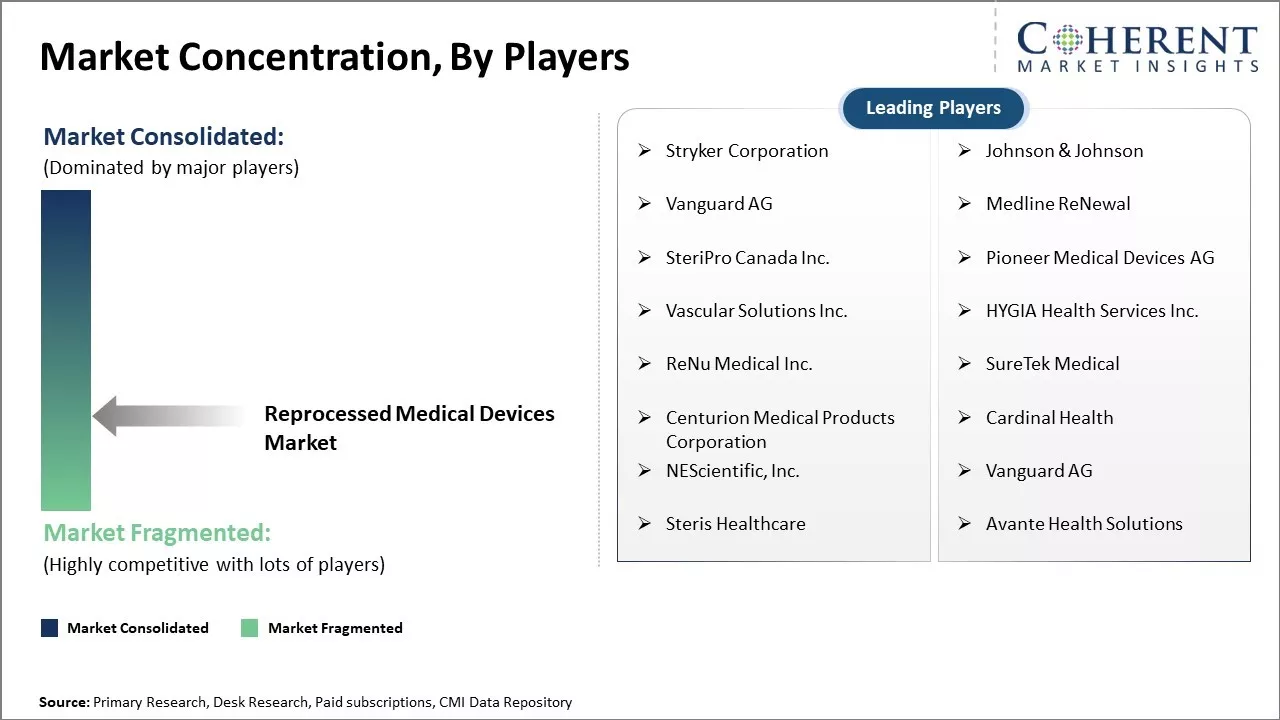
The type segment includes third-party and in-house. Third-party contributes the highest share of the reprocessed medical devices market and is projected to hold 75.3% of the market share in 2025. Reprocessing medical devices internally requires substantial infrastructure, trained personnel and quality certifications that increases costs for individual healthcare facilities. In contrast, third-party reprocessors have dedicated facilities, technical know-how, and strict regulatory guidelines in place to efficiently remanufacture devices. They can leverage volume to reduce turnaround times and offer competitive pricing for hospitals. Third-party services also eliminate the need for operator training and inventory management in individual centers. The complexity of reprocessing tasks like disassembly, cleaning, inspection, repair, and testing favors the reliance on expert third-party providers over in-house solutions. Strict regulatory oversight further makes the bar for self-clearance very high. Overall, outsourcing to well-established third-party reprocessors presents a more viable option for most healthcare facilities.
The end user segment includes hospitals & clinics, diagnostic centers, ambulatory surgical centers, and others. Hospitals & clinics contribute the highest share of the reprocessed medical devices market and is projected to hold 45.7% of the market share in 2025. Healthcare budgets remain under immense pressure due to rising medical costs and limited public funding in most countries. This has compelled providers to explore more affordable alternatives that do not compromise on quality and safety. Reprocessed devices offer pricing discounts of up to 50-70% compared to originals. The savings on device costs can then be channelized towards upgrading other hospital services or improving access through community outreach. Reuse also supports the ethical and environmental priority of extending device lifespan. Moreover, reprocessors have demonstrated that functionality and sterilization standards of refurbished devices are at par with new ones. This addresses a key concern among hospitals and eases adoption. The expansion of approved reprocessing services regionally also improves procurement availability for many healthcare facilities. Overall, cost savings and access to high-quality critical care continue driving hospitals to leverage reprocessed medical devices.
To learn more about this report, Download Free Sample
North America has established itself as the dominant player in the global reprocessed medical devices market and is expected to hold 49.9% of the market share in 2025. The region has a very well-developed medical devices sector with ample opportunities for reprocessing of devices. Most major players involved in reprocessing activities have their headquarters located in North America, allowing them to leverage the region's infrastructure and expertise. Moreover, the favorable regulations related to reprocessing in the US boosts investor confidence and new business openings in this space. Many hospitals and clinics also outsource their reprocessing needs to regional third-party reprocessors, ensuring continuous demand. With the rise in healthcare costs, reprocessed instruments provide an affordable alternative for facilities without compromising on quality. In 2020, U.S. hospitals saved $372 million by using reprocessed medical devices, which cost 25 to 40% less than new ones. Additionally, cutting down on medical waste led to even more savings. According to data from AMDR, if all hospitals adopted the reprocessing practices of the top 10% of hospitals, the total annual savings could have reached an extra $2.28 billion in 2020. This is further adding to the reprocessed medical device market demand.
Asia Pacific reprocessed medical devices market is experiencing significant growth. Countries like India, China, Japan, and South Korea are witnessing exponential growth opportunities. While import-reliant in nature initially, the presence of low-cost skilled labor and developing infrastructure has encouraged local manufacturing activities in recent years. Regional governments are supporting the sector through various initiatives in order to reduce healthcare spending and improve access to services. Southeast Asian countries have especially warmed up to the value proposition offered by reprocessed instruments. Burgeoning medical tourism coupled with rising income levels is expanding the customer base rapidly. Several international OEMs are also setting up local reprocessing units to target Asian markets and compete with global third-party reprocessors. With growing healthcare expenditures, cost-effective solutions, such as reprocessed devices, would become increasingly crucial for Asia Pacific to achieve universal health coverage goals.
The rising healthcare waste in United States is driving the adoption of reprocessed medical devices. The U.S. healthcare sector generates about 5 million tons of waste each year, which averages to around 29 pounds per hospital bed every day. Single-use devices (SUDs), commonly used in hospitals, account for nearly 80% of the healthcare industry’s carbon footprint through their production, transportation, usage, and disposal. Reprocessing medical devices can significantly reduce costs for hospitals and clinics by extending the lifecycle of expensive single-use devices.
To learn more about this report, Download Free Sample
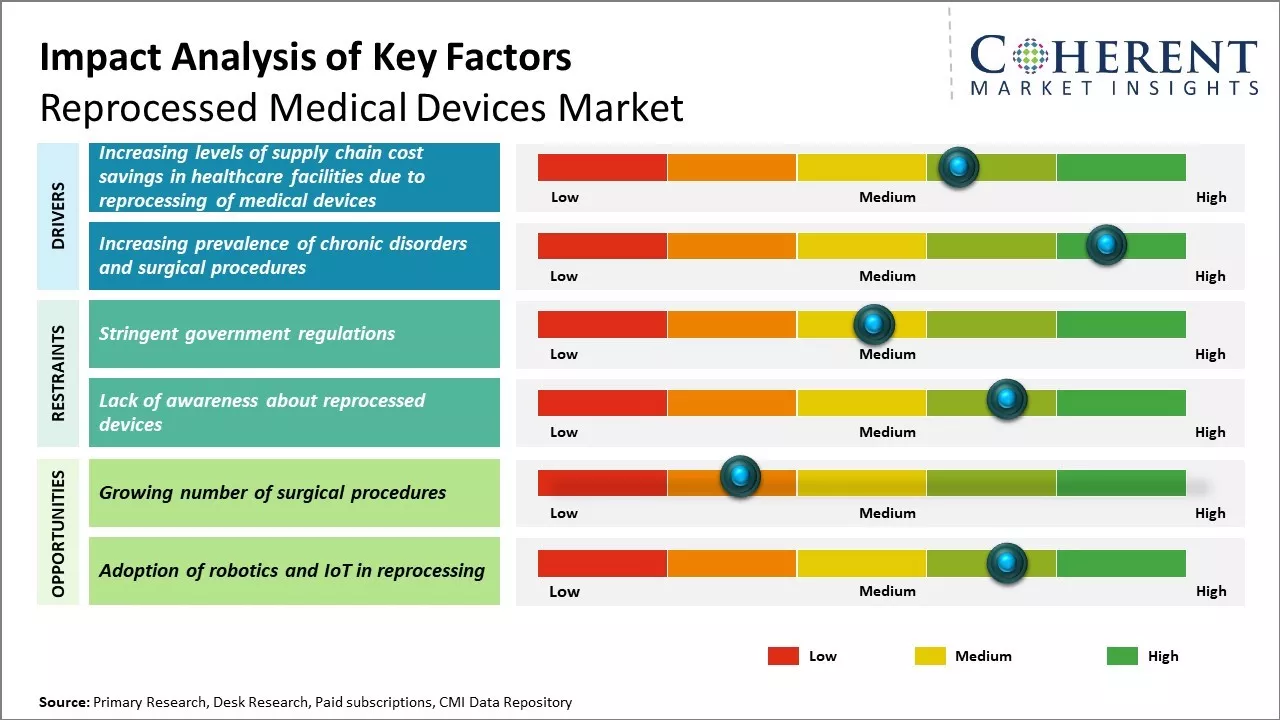
Increasing level of supply chain cost savings in healthcare facilities due to the reprocessing of medical devices are expected to drive growth of the reprocessed medical devices market. The use of reprocessed medical devices is expected to constantly growth, driven by increasing healthcare costs and the rising demand for cost-effective solution.
The increasing prevalence of chronic disorders is expected to drive the growth of the market during the forecast period. The forecast period's market dynamics are impacted by the increasing prevalence of chronic diseases. The market for medical device reprocessing is growing as a result of an increase in the prevalence of chronic diseases and surgical operations. For instance, according to data published in National Institutes of health (NIH), globally, over 310 million major surgeries are performed each year, around 40 - 50 million in USA and 20 million in Europe.
Rising healthcare costs and budget constraints have increased the demand for cost-effective alternatives like reprocessed devices. As baby boomers age, the demand for surgical procedures will rise, benefiting remanufacturers. Many devices are now designed for multiple uses, making reprocessing more feasible, thus creating lucrative growth opportunities for market development over the forecasted period.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 3.51 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 15.4% | 2032 Value Projection: | USD 9.57 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Stryker Corporation, Johnson & Johnson, Vanguard AG, Medline ReNewal , SteriPro Canada Inc., Pioneer Medical Devices AG, Vascular Solutions Inc., HYGIA Health Services Inc., ReNu Medical Inc., SureTek Medical, Centurion Medical Products Corporation, Cardinal Health, NEScientific, Inc., Vanguard AG, Steris Healthcare, and Avante Health Solutions |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: The reprocessed medical devices market consists of medical equipment and instruments that have been reprocessed or refurbished after use in hospitals and medical facilities. These devices include surgical instruments like forceps and retractors, endoscopes, orthopedic implants and components, and other equipment that can be cleaned, disinfected, sterilized, tested, repaired if necessary, and marketed to be used again by healthcare providers. Reprocessing helps reduce medical waste while providing more affordable device options for hospitals and patients.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients