Reprocessed medical devices are products that undergo reprocessing procedures and are used again. Reprocessing is a validated process used for a medical device that has been either used or is contaminated. Reprocessing of medical devices involves cleaning, disinfection, and sterilization.
Asia Pacific reprocessed medical devices market is estimated to be valued at US$ 376.2 million in 2021 and is expected to exhibit a CAGR of 15.3 % over the forecast period (2021-2028).
Recent Developments:
On May 31, 2021, PENTAX Medical (a division of HOYA Group) and Jiangsu Vedkang Medical Science and Technology Co., Ltd have established a joint venture- PENTAX Medical Therapeutics (Jiangsu) Co., Ltd. to develop single-use therapeutics products in the field of flexible medical endoscopy.
In October 2020, Olympus Corporation announced that it has launched the OERElite Automatic Endoscope Reprocessor (AER). It is designed to use AcecideC (a peracetic acid-based disinfectant) to clean and disinfect up to two endoscopes simultaneously in 28 minutes.
In April 2020, Cleanpart GmbH introduced a quality management system for medical devices according to DIN EN ISO 13485:2016.
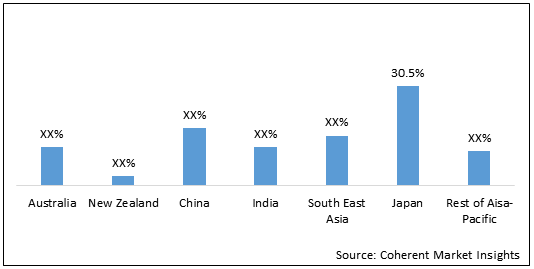
Figure 1. Asia Pacific Reprocessed Medical Devices Market Value (US$ Mn), by Country, 2020
To learn more about this report, Download Free Sample
Japan held dominant position in Asia Pacific reprocessed medical devices market in 2020, accounting for 30.5% share in terms of volume, followed by China and South East Asia, respectively. The growth of the market is driven by growing adoption, and high unmet medical needs are expected to boost the market growth in Japan.
Asia Pacific Reprocessed Medical Devices Market: Drivers
Increasing focus on reducing medical waste is expected to propel growth of Asia Pacific reprocessed medical devices market over the forecast period. For instance, according to the National Bureau of Statistics of China, Over 2 million tons of medical waste were produced in China in 2018.
Moreover, use of reprocessed medical devices leads to significant savings in the healthcare sector, which is expected to boost demand for such devices, thereby aiding in growth of the market. For instance, according to Association of Medical Device Reprocessors, reprocessing medical devices originally labeled for single use saved hospitals and surgery centers nearly US$ 500 million in 2018.
Asia Pacific Reprocessed Medical Devices Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2020 | Market Size in 2021: | US$ 376.2 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2021 to 2028 |
| Forecast Period 2021 to 2028 CAGR: | 15.3% | 2028 Value Projection: | US$ 1,235.5 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Stryker Sustainability Solutions, Inc., Medline ReNewal, Hygia Health Services, Inc., Cleanpart GmbH, ReNu Medical, Inc., SureTek Medical, and NEScientific |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Asia Pacific Reprocessed Medical Devices Market: Opportunities
Reduced product pricing of reprocessed medical devices is expected to offer lucrative growth opportunities for players in the market. Consumers in economies such as India, China, Indonesia, and Malaysia are very price conscious. In these countries, reprocessed medical devices are sold at approximately half the price of a new device. Therefore, products with less price and appropriate regulatory approval would be adopted faster in the region.
Moreover, strengthening distribution network is also expected to aid in growth of the market. Increasing population and high unmet medical needs are expected to boost the market growth in Asia Pacific. Market players, therefore, need to focus on enhancing their distribution network to remain competitive in the market.
Market Trends:
Major medical device manufacturers are focused on investment in reprocessed medical devices. For instance, in April 2019, NEScientific Inc. and ReNu Medical, Inc. joined The Association of Medical Device Reprocessors. Similarly, in November 2018, Cardinal Health also joined the association.
Sterile processing is expected to aid in growth of the market. Investment in staffing for central sterile departments staff is expected to reduce medical waste in the healthcare sector. Central sterile professionals can maximize their single use device reprocessing leading to significant saving.
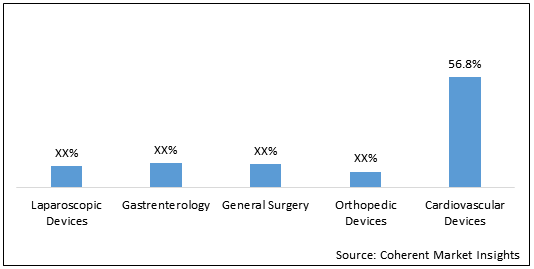
Figure 2. Asia Pacific Reprocessed Medical Devices Market Share, By Technique, 2020
To learn more about this report, Download Free Sample
Key Takeaways of the Graph:
Cardiovascular Devices held dominant position by Technique in the market and accounted for 56.8% share in the Asia Pacific reprocessed medical devices market in 2020. The segment is expected to reach US$ 863.9 million in 2028. Growth of the segment is driven by higher adoption of cardiovascular devices, increasing prevalence of cardiovascular diseases, and aging population prone to cardiac diseases. For instance, as per National Crime Records Bureau (NRCB), India, deaths due to heart attacks has been increased by 53% in 5 years in
India from 2014 to 2019.
Asia Pacific Reprocessed Medical Devices Market: Restraints
Risk of cross contamination and hospital acquired infections is expected to hinder growth of Asia Pacific reprocessed medical devices market. Absence of stringent regulations for reprocessed medical devices in developing countries may result in increased cases of contamination and infections due to the use of these devices.
Moreover, material alteration during exposure of device to extreme environment is also expected to limit growth of the market. Exposure to certain chemical agents such as decontamination agents and chemical sterilants during reprocessing procedures can damage medical devices. Residues from contamination agent materials or chemicals, if absorbed, can create chemical burns or cause sensitization in patients.
Asia Pacific Reprocessed Medical Devices Market: Competitive Landscape
Major players operating in Asia Pacific reprocessed medical devices market include, Stryker Sustainability Solutions, Inc., Medline ReNewal, Hygia Health Services, Inc., Cleanpart GmbH, ReNu Medical, Inc., SureTek Medical, and NEScientific.
Asia Pacific Reprocessed Medical Devices Market: Key Developments
Major players in the market are focused on adopting M&A strategies to expand their product portfolio. For instance, in 2018, Stryker Corporation acquired the U.S.-based Hygia Health Services Inc., a company focused into reprocessing patient care single-use devices.
Similarly, in 2018, the U.S.-based Arjo acquired ReNu Medical Inc, a company that specializes in green reprocessing for single use non-invasive medical devices.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients