Ragweed Pollen Allergy Treatment Market is estimated to be valued at USD 1,044.8 Mn in 2025 and is expected to reach USD 1,431.4 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 4.6% from 2025 to 2032.
Analysts’ views on global ragweed pollen allergy treatment market:
Increasing prevalence of chronic diseases, new product launches, and strategies like mergers, acquisitions, and collaboration are expected drive the global ragweed pollen allergy treatment market growth over the forecast period. For instance, according to the data published by the World Health Organization, on May 4, 2023, in 2022, percent of adults who have ever been diagnosed with chronic obstructive pulmonary disease (COPD), emphysema, or chronic bronchitis was 4.6% while number of visits to emergency departments with COPD as the primary diagnosis were nearly 1.2 million. Number of deaths with chronic lower respiratory disease including asthma was 142,342. Pulmonary diseases are generally result of chronic allergies. Hence this increasing pulmonary diseases will drive the global ragweed pollen allergy treatment market growth over the forecast period.
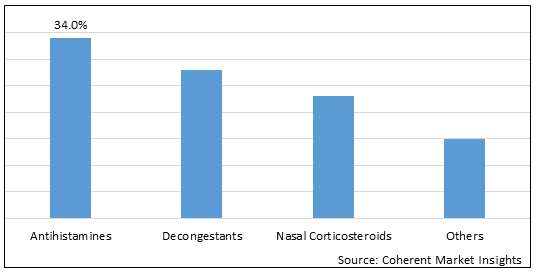
Figure 1. Global Ragweed Pollen Allergy Treatment Market Share (%), By Drug Class, 2025
To learn more about this report, Download Free Sample
Global Ragweed Pollen Allergy Treatment Market– Drivers
Increasing research & development activities in ragweed pollen allergy treatment
Increasing research & development activities in ragweed pollen allergy treatment is expected to drive the global ragweed pollen allergy treatment market growth over the forecast period. For instance, on July 2, 2022, Stallergenes Greer, a Switzerland-based global healthcare company specializing in the diagnosis and treatment of allergies through the allergy immunotherapy (AIT) products, announced positive data from its EfficAPSI real-world study. Presented at the 2022 European Academy of Allergy and Clinical Immunology (EAACI) congress in Prague (Czech Republic), the real-world study confirmed significant benefit of sublingual liquid allergen immunotherapy treatment (AIT) on the onset and worsening of asthma in patients with allergic rhinitis. The retrospective longitudinal pharmaco-epidemiological real-world study included over 430,000 patients more than 100,000 patients with allergic rhinitis with or without asthma treated with sublingual liquid immunotherapy and symptomatic drugs; compared to more than 330,000 patients with allergic rhinitis with or without asthma treated with symptomatic drugs only. The results showed a reduction of the risk of asthma onset of more than 20% observed in patients undergoing treatment with sublingual liquid AIT and symptomatic drugs versus patients treated with symptomatic drugs only; and a reduction of the risk of asthma worsening of 28% and reaching 37% for severe forms.
Growing number of geriatric population
Increasing number of geriatric population is expected to drive the global ragweed pollen allergy treatment market growth. Due to the compromised immune systems, geriatrics are more susceptible to get various types of allergies, further estimated to enhance the market’s growth rate. For instance, according to the data shared by the World Health Organization, on October 1, 2022, in 2020, the number of people aged 60 years and older outnumbered children younger than 5 years. By 2030, 1 in 6 people in the world will be aged 60 years or over. At this time the share of the population aged 60 years and over will increase from 1 billion in 2020 to 1.4 billion. By 2050, the world’s population of people aged 60 years and older will double (2.1 billion). The number of persons aged 80 years or older is expected to triple between 2020 and 2050 to reach 426 million.
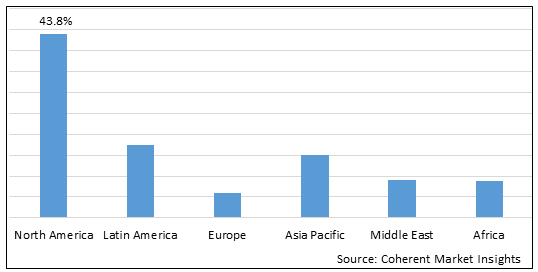
Figure 2. Global Ragweed Pollen Allergy Treatment Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
Global Ragweed Pollen Allergy Treatment Market- Regional Analysis
Among region, North America is estimated to hold a dominant position in the global ragweed pollen allergy treatment market over the forecast period, owing to increasing launches of products. For instance, according to an article published by the Current Opinion in Allergy and Clinical Immunology on December 3, 2021, scientist at Johns Hopkins School of Medicine, Division of Allergy, Immunology, and Rheumatology, a U.S. based public research university suggested that when combined with allergen immunotherapy the biologic omalizumab (Xolair) can enhance efficacy for the treatment of allergic rhinitis, venom hypersensitivity, and food allergy, and decrease adverse reactions.
Global Ragweed Pollen Allergy Treatment Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, the U.A.E., Egypt, and others faced problems regarding transportation of drugs from one place to another.
However, the COVID-19 pandemic had positive impact on the global ragweed pollen allergy treatment market, due to increased demand of antihistamines in treatment of COVID infection. For instance, according to an article published by the Helion on May 9, 2023, The National Institute of Health (NIH) recommends the use of over-the-counter antipyretics, analgesics, or antihistamines for the management of symptoms for outpatients COVID-19 patients. They are a well-known class of drugs commonly used in primary care. Several second generation antihistamines have a good safety profile when dosed up to four times the standard dose. Commonly prescribed antihistamines include cetirizine, loratadine, ebastine etc. It has been hypothesized that antihistamines help damped mast cell degranulation, resulting in a reduction of the cytokine storm observed in severe COVID-19 disease.
Ragweed Pollen Allergy Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1,044.8 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 4.6% | 2032 Value Projection: | USD 1,431.4 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
ALK-Abello A/S, ASIT Biotech SA, Anergis SA, Biomay AG, Astellas Pharma Inc., Thermo Fisher Scientific Inc, Siemens Healthcare Private Limited, Omega Diagnostics Group PLC, Stallergenes Greer, bioMerieux SA, Lincoln Diagnostics, Inc, HOB Biotech Group Corp Ltd, HYCOR Biomedical, Inc, Alcon, Hitachi Chemical Diagnostics, Inc, Quest Diagnostics Incorporated, Circassia, Immunomic Therapeutics, Inc, Novartis AG, Japan Tobacco Inc, GlaxoSmithKline plc, REGiMMUNE Co, Ltd, Sanofi, and Merck KGaA. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Ragweed Pollen Allergy Treatment Market Segmentation:
Global ragweed pollen allergy treatment market report is segmented into drug class, distribution channel, and region.
Based on drug class, the global ragweed pollen allergy treatment market is segmented into antihistamines, decongestants, nasal corticosteroids, and others. Out of which, the antihistamines segment is expected to dominate the market due to increasing launch of these category of drug as anti-allergic medication.
Based on distribution channel, the global ragweed pollen allergy treatment market is segmented into hospital pharmacy, retail pharmacy, and online channel. Among these, hospital pharmacy segment is expected to dominate the market over the forecast period due to increasing numbers of hospital pharmacies.
Based on region, the global ragweed pollen allergy treatment market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa. Among these, North America segment is expected to dominate the market over the forecast period due to increasing research & development in this region.
Among all segmentation, the drug class segment has the highest potential due to increasing launches of products by the key market players. For instance, on January 14, 2022, U.S. Food and Drug Administration, approved Ryaltris Nasal Spray for seasonal allergic rhinitis. The product is approved for patients above aged 12 or older. The product is manufactured by Glenmark Pharmaceuticals Ltd., an India-based multinational pharmaceutical company. Ryaltris will be marketed and distributed in the U.S. by Hikma Specialty U.S.A., Inc., a U.S.-based pharmaceutical company, as part of its exclusive licensing agreement with Glenmark Specialty S.A (Switzerland).
Global Ragweed Pollen Allergy Treatment Market Cross Sectional Analysis:
Introduction of newer products in allergic treatment by key market players in Europe region is expected to drive growth of drug class segment in the region. For instance, in September 2021, Stallergenes Greer, a Switzerland-based global healthcare company specializing in the diagnosis and treatment of allergies through the allergy immunotherapy (AIT) products, launched Orylmyte, sublingual immunotherapy tablet for treatment of allergies in Germany.
Global Ragweed Pollen Allergy Treatment Market: Key Developments
On March 17, 2022, Perrigo Company plc, an Ireland-based over-the-counter consumer goods and specialty pharmaceutical company, announced that it has received final approval from the U.S. Food and Drug Administration for the over-the-counter use of Nasonex 24hr Allergy (mometasone furoate monohydrate 50mcg) for treatment of allergic rhinitis. This approval marks the first branded Rx-to-OTC switch for the company.
On July 29, 2021, GSK plc., a U.K.-based multinational pharmaceutical and biotechnology company, announced that the U.S. Food and Drug Administration (FDA) has approved Nucala (mepolizumab), a monoclonal antibody that targets interleukin-5 (IL-5), as a treatment for patients with chronic rhinosinusitis with nasal polyps (CRSwNP). This new indication for mepolizumab is for the add-on maintenance treatment of CRSwNP in adult patients 18 years of age and older with inadequate response to nasal corticosteroids.
On February 5, 2020, Xencor, a U.S.-based clinical-stage biopharmaceutical company developing engineered monoclonal antibodies for the treatment of cancer and autoimmune diseases, announced it has granted an exclusive worldwide license to develop and commercialize the investigational humanized monoclonal antibody XmAb 7195 to Aimmune Therapeutics, a U.K. based biopharmaceutical company. XmAb7195, which has been renamed AIMab7195, was originally developed by Xencor for the treatment of allergic asthma. It uses three distinct mechanisms of action to reduce blood serum IgE and suppress IgE-producing cells.
On July 18, 2022, Nestlé Health Science, a Switzerland-based nutritional science company invested US$ 41 million to co-develop Enterome’s preclinical IL-10 inducer EB1010, molecule designed to treat allergic reactions in gut and collaborate on the discovery of other allergy candidates. Enterome is France based clinical stage biotechnology company.
Global Ragweed Pollen Allergy Treatment Market: Key Trends
Strategies like expansion by key market players
Introduction of strategies like expansion by key market players can drive growth of market. For instance, on July 12, 2022, Jerath Path Labs, an India-based pathology lab and chain of allergy testing, announced plan of international expansion with launch of new franchises and allergy treatment centers in Africa. The expansion is with aim of providing comprehensive, high-quality, rapid-response laboratory testing at affordable prices.
Launch of products by key market players
Introduction of new products by key market players can drive growth of market. On January 25, 2023, ALK, a Denmark-based pharmaceutical company which specializes in the development and manufacture of allergy immunotherapy (AIT) products for the prevention and treatment of allergy, announced that the U.S. Food and Drug Administration (FDA) approved ODACTRA Tablet for Sublingual Use for the treatment of allergic rhinitis in persons ages 12 through 17.
Global Ragweed Pollen Allergy Treatment Market: Restraints
Poor adherence to allergy treatments
The poor adherence to allergy treatments is expected to hamper the global ragweed pollen allergy treatment market growth. For instance, according to an article published by the Journal Frontiers in Allergy on January 5, 2022, study was performed to analyse dropout rates from subcutaneous immunotherapy (SCIT) for allergy treatment. A total of 719 patients were studied out of which 91% patient’s discontinued sublingual immunotherapy (SLIT). It was concluded that the inconvenience caused by SCIT and the adverse effects associated with subcutaneous and sublingual therapy are the main reasons for discontinuing these therapies among patients.
To counterbalance this restraint, patient counselling should be done to encourage them to adhere to treatment.
High cost of allergy treatments
In emerging economies, high cost of allergy treatments is expected to hamper the global ragweed pollen allergy treatment market growth. For instance, according to an article published in Journal Current Medical Research and Opinion on April 2, 2021, the cost of allergy shot is about US$ 1,600 to US$ 4,000 per year for no insurance coverage. It is around US$ 800 per year for patients with proper reimbursement. However the SLIT drops are not eligible for reimbursement. Such high costs can hamper growth of market.
To counterbalance this restrain, reimbursement policies should be introduced.
Global Ragweed Pollen Allergy Treatment Market - Key Players
Major players operating in the global ragweed pollen allergy treatment market include ALK-Abello A/S, ASIT Biotech SA, Anergis SA, Biomay AG, Astellas Pharma Inc., Thermo Fisher Scientific Inc, Siemens Healthcare Private Limited, Omega Diagnostics Group PLC, Stallergenes Greer, bioMerieux SA, Lincoln Diagnostics, Inc, HOB Biotech Group Corp Ltd, HYCOR Biomedical, Inc, Alcon, Hitachi Chemical Diagnostics, Inc, Quest Diagnostics Incorporated, Circassia, Immunomic Therapeutics, Inc, Novartis AG, Japan Tobacco Inc, GlaxoSmithKline plc, REGiMMUNE Co, Ltd, Sanofi, and Merck KGaA.
Global Ragweed Pollen Allergy Treatment Market– Definition
Ragweed pollen is considered as one of the most common roots of seasonal allergies, especially in the U.S. The pollen present in ragweed plants is the main cause of this disease. These plants are found in 17 different types of species in North America. An autoimmune response is generated in ragweed allergy affected population. The immune cells of affected patients produces chemicals to combat the pollen considering it as a foreign substance. This reaction leads to various irritating symptoms, such as itchy eyes, running nose, and sneezing. However, these symptoms or allergy can be treated with allergy shots and medications.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients