Global allergy immunotherapy market is estimated to be valued at USD 2,740.6 Mn in 2025 and is expected to reach USD 5,276.5 Mn by 2032, exhibiting a compound annual growth rate (CAGR) of 9.8% from 2025 to 2032.
To learn more about this report, Download Free Sample
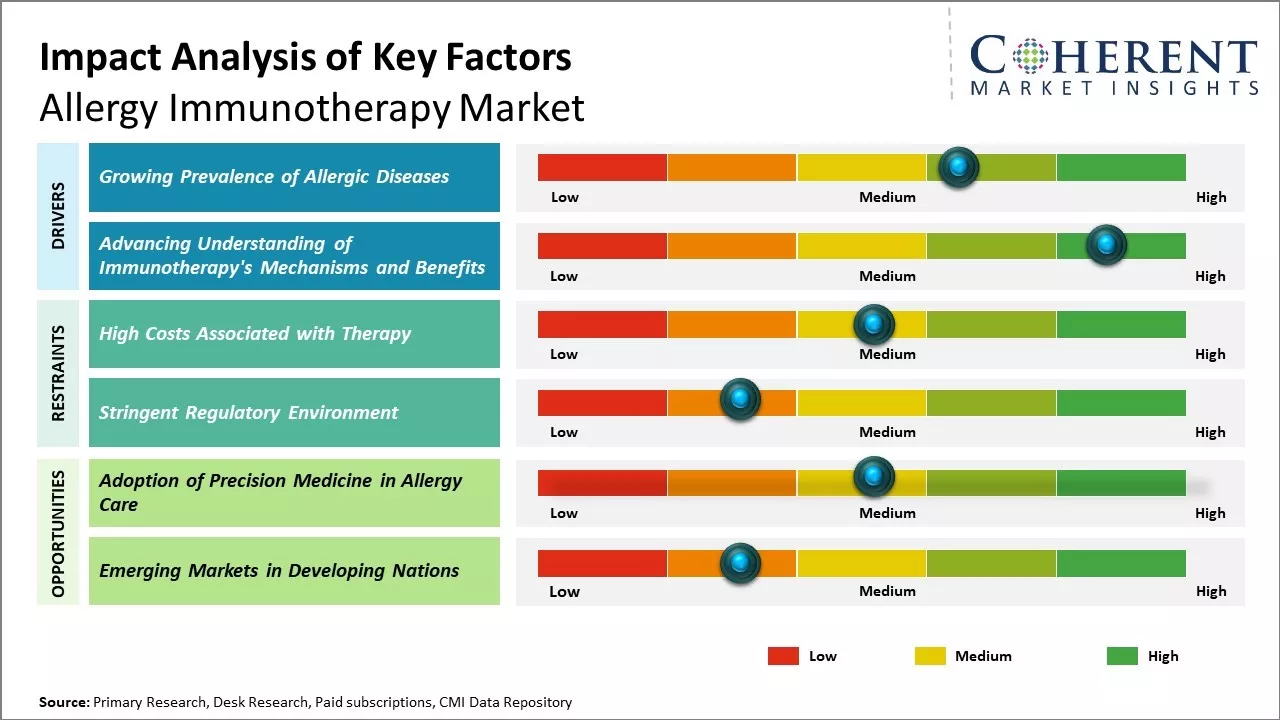
The Global Allergy Immunotherapy Market is expected to grow significantly during the forecast period due to the increasing cases of allergies. Public allergies pose a challenge to the healthcare system, as they are now a widespread illness, impacting people of all ages around the globe. A case in point, the American College of Allergy, Asthma, and Immunology ACAAI has reported that the United States has around 6% of adults and 8% of children suffering from food allergies. Appreciable growth, along with the prevailing burden of such chronic diseases, is expected to increase the need for safe and effective healthcare solutions, thereby propelling the market growth.
|
Event |
Description and Impact |
|
Regulatory Harmonization and Approval Acceleration Initiatives |
|
|
Climate Change and Environmental Allergen Pattern Shifts |
|
|
Technological Breakthroughs in Personalized Medicine |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
To learn more about this report, Download Free Sample
In terms of Treatment Type, Tetrahydrocannabinol (THC) sub-segment is estimated to hold 53.3% of the market share in 2025, owing to its enduring efficacy and simplicity. SCIT involves administering graded doses of allergen extract under the skin, building tolerance slowly over time.
This gradual, prolonged exposure allows the body to develop a tolerance and reduces the risk of severe allergic reactions that can occur with other treatments. Long-term studies show patients often experience symptom relief and reduce their medication usage for 3-5 years after completion of treatment. Its predictable response provides reassurance to both physicians and patients.
Despite requiring weekly visits initially, compliance tends to be high because results materialize gradually over months of therapy versus sudden effects that could raise safety concerns. The straightforward delivery method of SCIT provides it an advantage. Administered in a doctor's office, it presents minimal burden on patients compared to daily at-home therapies.
This simplicity boosts adherence and helps maximize treatment impact. It also allows for close monitoring by trained staff to promptly address any adverse reactions. Due to these risk-management advantages, SCIT remains the standard of care recommended in clinical practice guidelines for most common allergies like dust mites and pollen.
In terms of Allergy Type, the allergic asthma sub-segment is estimated to hold 36.6% of the market share in 2025, owing to the rising prevalence of asthma globally.
According to recent estimates by health organizations, over 300 million people suffer from asthma worldwide. The condition is characterized by inflammation of the airways and difficulty in breathing. Conventional treatment of acute asthma involves the use of short-acting beta-agonists to relax bronchial muscles. However, long-term control medications such as inhaled corticosteroids and leukotriene modifiers are essential to prevent recurrent symptoms.
The asthma segment is driven by the need for more effective medicines that can better manage symptoms over prolonged use. Several drug makers are investing in research to develop novel biologic drugs targeting specific inflammatory pathways involved in asthma pathophysiology.
Monoclonal antibody therapies that block immune system proteins like IgE and IL-5 have shown promising results in clinical trials. Such targeted drugs offer superior efficacy with less risk of side effects compared to existing options. Their approval and commercialization in major markets will provide a significant boost to the asthma segment in the coming years.
In terms of Distribution Channel, The hospital pharmacies sub-segment is estimated to hold 51.7% of the market share in 2025, due to their accessibility and promotion of services. As the traditional source for allergy treatments, hospitals provide convenient access for individuals seeking immunotherapy evaluation and ongoing treatment.
Being located in most major medical areas, hospitals present minimal transportation hurdles for patients. Their extensive specialty clinics and on-site pharmacies allow for streamlined evaluations, prescriptions and dispensing within one facility. Patients appreciate the integrated 'one-stop-shop' for their care.
Major hospitals also spearhead advancement in immunotherapy through dedicated research departments. Their position at the forefront of new protocol developments and clinical trials increases patients' confidence in outcomes. This reputation cements hospitals' leadership role and widens their reach, sustaining their position as the primary channel supplying allergy immunotherapy. With optimized logistics and continuous promotion, hospitals are optimally equipped to capture the highest market share.
To learn more about this report, Download Free Sample
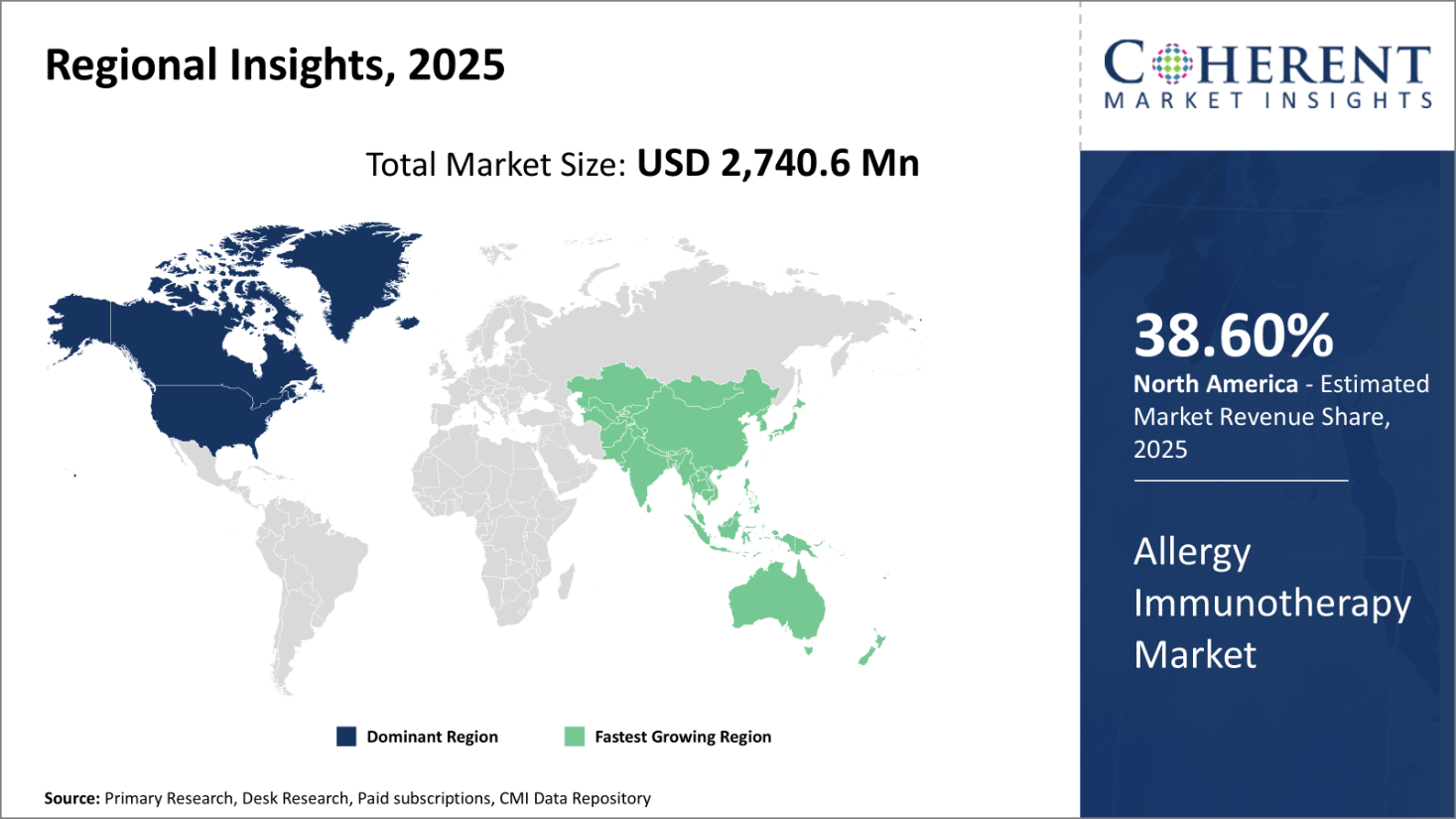
North America is expected to maintain its leadership position in the global allergy immunotherapy market in 2025, accounting for an estimated 38.60% share of the total market. This dominance is largely attributed to the strong presence of leading pharmaceutical and biotechnology companies across the U.S. and Canada, which continue to drive innovation and product development through robust R&D activities.
Supportive reimbursement frameworks and insurance coverage for immunotherapy procedures have significantly increased patient access and provider adoption rates. Furthermore, North America boasts a mature healthcare infrastructure, facilitating the widespread use of advanced immunotherapy treatments across diverse patient populations.
The region also benefits from a well-established network of manufacturing facilities that serve both domestic and international markets. This enables efficient supply chain management, timely delivery of products, and responsiveness to shifting demand patterns. The availability of a broad portfolio of immunotherapy products for various allergens further reinforces North America’s competitive edge in the global market.
Asia Pacific has emerged as the fastest-growing region in the global allergy immunotherapy market, driven by a surge in allergy cases and rising healthcare awareness across developing economies such as India, China, and Southeast Asia. Rapid economic growth, increasing healthcare spending, and expanding access to medical services are fueling the demand for effective allergy treatments.
The region’s momentum is bolstered by favorable government initiatives aimed at strengthening local pharmaceutical manufacturing and improving regulatory frameworks. Collaboration between domestic players and global pharmaceutical companies has accelerated the transfer of knowledge and technology, enhancing the quality and availability of immunotherapy solutions.
Additionally, the region’s large patient base, coupled with growing investments in healthcare infrastructure, presents significant opportunities for market expansion. Asia Pacific’s trajectory is marked by long-term potential as local firms scale up production capabilities and broaden their product offerings to meet evolving medical needs.
China leads the Asia Pacific allergy immunotherapy market, driven by a rapidly rising prevalence of allergic conditions and strong national focus on improving healthcare access. The country’s expanding middle class, urbanization, and increasing environmental pollution contribute to a growing patient population in need of advanced allergy treatments.
Government initiatives promoting domestic biopharmaceutical innovation, along with favorable regulatory reforms, are accelerating the development and approval of immunotherapy products. China’s extensive manufacturing base, combined with strategic collaborations with global pharmaceutical leaders, enhances its capacity to produce a broad range of allergy immunotherapy solutions at scale.
Additionally, the integration of immunotherapy into public health programs and hospital formularies is supporting wider adoption, particularly in urban centers. These factors position China as a key growth driver in the regional and global allergy immunotherapy landscape.
India is emerging as a high-growth market in the Asia Pacific allergy immunotherapy sector, fueled by a sharp rise in allergic diseases and increasing public health awareness. Rapid urbanization, environmental changes, and changing lifestyles have led to a surge in allergic rhinitis, asthma, and food allergies across the country.
The Indian government’s efforts to improve healthcare delivery through programs like Ayushman Bharat and the promotion of local pharmaceutical manufacturing under the “Make in India” initiative are creating a conducive environment for allergy treatment expansion. Indian firms are increasingly partnering with global companies to gain access to immunotherapy technologies and expertise.
Rising investments in diagnostic infrastructure and specialty clinics, alongside a growing base of trained allergists, are boosting access to immunotherapy in tier-1 and tier-2 cities. As affordability improves and awareness spreads, India is poised to become a major contributor to the region’s allergy immunotherapy market growth.
Japan plays a strategic role in the Asia Pacific allergy immunotherapy market, anchored by its advanced medical infrastructure and high standards of clinical care. The country has a well-documented burden of allergic conditions such as cedar pollen allergy and food-related sensitivities, driving consistent demand for targeted treatments.
Japan’s commitment to research excellence and precision medicine supports the development of innovative immunotherapy solutions, often tailored to specific allergens prevalent in the local environment. The country’s stringent regulatory framework ensures high product safety and efficacy standards, fostering trust among healthcare providers and patients alike.
With a strong emphasis on preventive care and early diagnosis, Japan continues to integrate allergy immunotherapy into its broader healthcare strategies. This, along with its aging population and high health consciousness, sustains steady market demand and positions Japan as a hub for technological advancement in allergy treatment.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2,740.6 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.8% | 2032 Value Projection: | USD 5,276.5 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
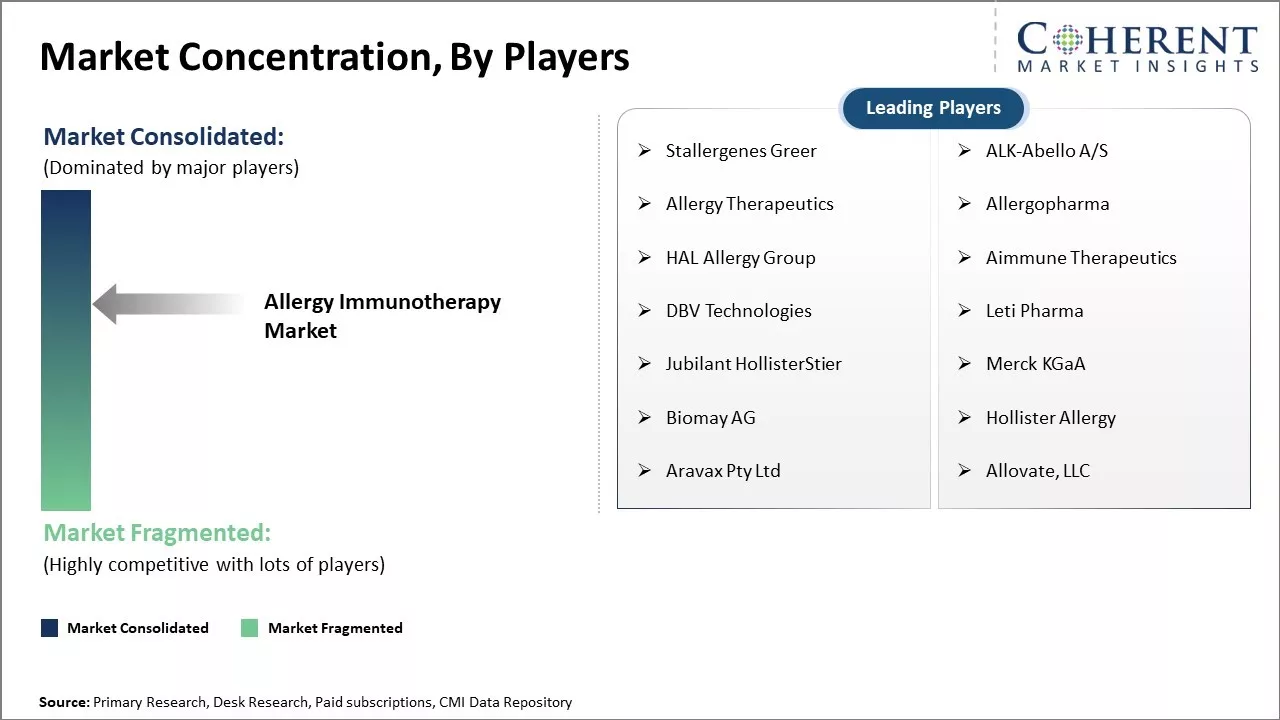
Stallergenes Greer, ALK-Abello A/S, Allergy Therapeutics, Allergopharma, HAL Allergy Group, Aimmune Therapeutics, DBV Technologies, Leti Pharma, Jubilant HollisterStier, Merck KGaA, Biomay AG, Hollister Allergy, Aravax Pty Ltd, Allovate, LLC |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The increasing availability of allergen immunotherapy solutions is one of the factors that is expected to drive the market growth during the forecast period. For instance, in September 2025, Regeneron announced successful Phase 3 trials for two investigational allergy therapies targeting grass and birch pollen. The treatments, developed with Sanofi, significantly reduced allergic symptoms, marking a major step toward expanding biologic-based allergen immunotherapy options. These results support Regeneron’s strategy to diversify its pipeline and address unmet needs in seasonal allergy care.
Basic and clinical research over the past decade has significantly expanded knowledge of how allergen immunotherapy works at the molecular level to provide long-lasting relief. Its complex effects on immune cells, cytokines and antibodies responsible for modifying the body's response to allergens are now increasingly well characterized.
This growing scientific evidence base is enabling refinement and targeting of immunotherapy protocols. It is also translating to demonstrable real-world advantages over existing symptomatic therapies which treat only a fraction of patients successfully. Studies following thousands of patients for many years now prove immunotherapy can prevent new sensitizations from developing in addition to treating existing ones.
Multiple long-term outcome studies also confirm that its benefits endure for 3-5 years after discontinuation of treatment. This disease-modifying impact sets it apart and raises expectations of altering the natural history of allergy in susceptible individuals.
Increasing research and development activities by market players is expected to offer lucrative growth opportunities over the forecast period. For instance, in August 2023, Inimmune Corporation (“Inimmune”), a clinical-stage company focused on the development of innovative immunotherapeutics, announced that the first subject has been dosed in Inimmune’s phase 1/1b First-in-Human clinical trial of INI-2004, Inimmune’s TLR4 agonist product candidate under development as a potential treatment of allergic rhinitis.
The phase 1/1b trial is a randomized, double-blind, placebo-controlled, dose-escalating study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of INI-2004 in healthy volunteers and participants with allergic rhinitis (AR).
Definition: Allergy immunotherapy refers to treatments that help desensitize people to specific allergens like dust, pollen, animal dander, bee venom, or food, through controlled doses of the allergen, administered over months or years, to gradually reduce sensitivity and symptoms. It works by exposing the immune system to small but increasing amounts of an allergen to retrain it not to overreact.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients