Prophylactic Human Vaccine Market Size and Forecast – 2025 – 2032
The Global Prophylactic Human Vaccine Market size is estimated to be valued at USD 72.8 billion in 2025 and is expected to reach USD 115.3 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 6.8% from 2025 to 2032.
Global Prophylactic Human Vaccine Market Overview
Prophylactic human vaccines are biological preparations designed to stimulate an immune response that prevents infectious diseases before exposure to pathogens. These products consist of inactivated, attenuated, subunit, conjugate, or mRNA-based formulations that introduce antigenic components of viruses or bacteria to the immune system. Over the years, vaccine development has advanced from traditional egg-based production to recombinant DNA and messenger RNA platforms, enhancing speed and scalability.
Key Takeaways
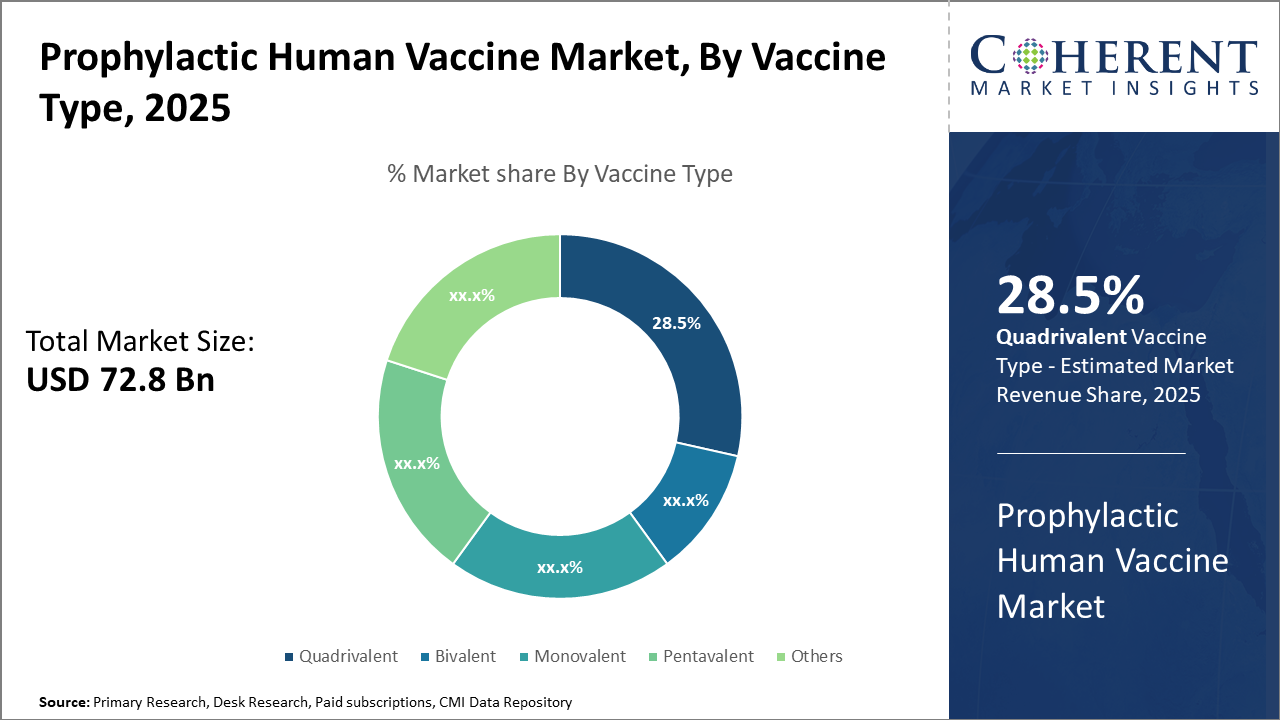
The Quadrivalent vaccine type leads market share with 28.5%, indicating broad acceptance due to enhanced protection against multiple flu strains. Recombinant vaccine technology is the fastest-growing platform, driven by recent successful launches contributing to expanding market revenue streams.
Pediatric immunization applications dominate, underpinning continuous demand thanks to extensive childhood vaccination programs globally.
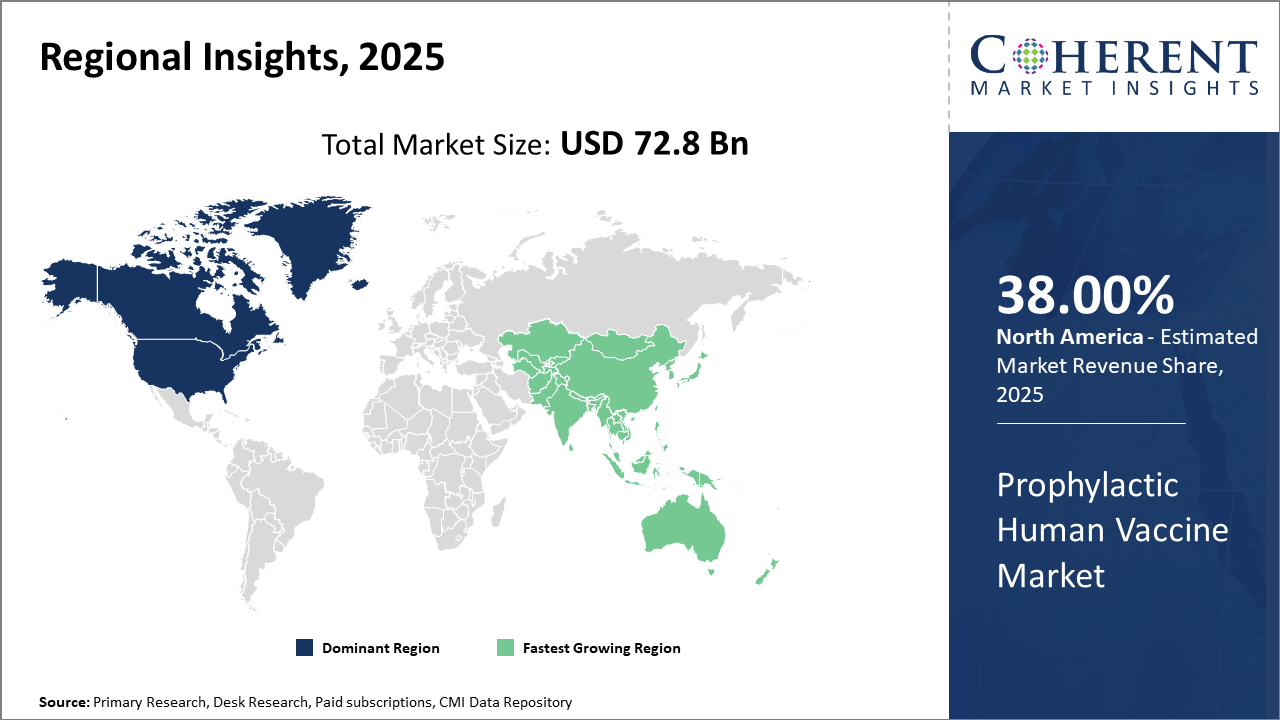
Regionally, North America holds the largest industry share with 38%, spearheaded by strong government health policies and the presence of leading market companies facilitating innovation.
Asia Pacific exhibits the fastest CAGR, underpinned by rising healthcare expenditure, expanding immunization coverage in populous countries such as India and China, and favorable regulatory frameworks.
Europe remains a critical hub for advanced vaccine technologies and stringent safety regulations, enhancing market credibility.
Prophylactic Human Vaccine Market Segmentation Analysis
To learn more about this report, Download Free Sample
Prophylactic Human Vaccine Market Insights, By Vaccine Type
Quadrivalent dominates the market share at 28.5%. The Quadrivalent vaccine's dominance stems from its broad protection against multiple influenza strains, making it the preferred choice in immunization schedules. Pentavalent vaccines are the fastest-growing subsegment, driven by emerging markets adopting combination vaccines to simplify immunization programs and ensure coverage across multiple diseases.
Prophylactic Human Vaccine Market Insights, By Technology Platform
Recombinant vaccines currently lead growth trends as cutting-edge biotechnology enables enhanced antigen presentation and safety profiles. The mRNA-based platform, having emerged prominently during the pandemic response, is exhibiting aggressive growth due to its adaptability and rapid scalability. Live Attenuated vaccines retain steady market demand for certain viruses like measles and rubella, while Inactivated vaccines continue to be essential in traditional immunization programs.
Prophylactic Human Vaccine Market Insights, By Application
Pediatric immunization commands a significant share due to extensive global initiatives targeting childhood disease prevention. Adult vaccination is the fastest-growing subsegment, supported by increasing awareness regarding influenza, HPV, and shingles vaccines among adults. Geriatric vaccination is gradually expanding, addressing aging populations with specific preventive healthcare needs.
Prophylactic Human Vaccine Market Trends
The Prophylactic Human Vaccine market is undergoing transformative changes driven primarily by technological advancements and strategic collaborations.
The introduction of mRNA vaccine platforms, which saw rapid development, continues to revolutionize vaccine research, thereby propelling extensive pipeline activities and regulatory approvals in 2024.
This trend has impacted other vaccine segments, enhancing immunogenicity while shortening development timelines. Additionally, adoption of combination vaccines is increasing globally, supported by data demonstrating improved patient compliance and reduced logistical costs.
For example, the Asia Pacific experienced a substantial shift toward pentavalent and quadrivalent formulations in 2024, driven by government initiatives focused on immunization coverage expansion.
Furthermore, digital innovations such as blockchain for cold chain tracking enhance supply chain transparency and vaccine integrity, notably in North America and Europe, thus shaping a more secure and efficient market ecosystem.
Prophylactic Human Vaccine Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Prophylactic Human Vaccine Market Analysis and Trends
In North America, the market's dominance in the Prophylactic Human Vaccine market stems from a highly developed healthcare infrastructure, significant government funding for vaccine programs, and the presence of leading market companies such as Pfizer and Moderna. The region accounts for approximately 38% of the industry share, driven by continuous innovation and accelerated vaccine approval processes. U.S. CDC programs supporting adult and pediatric vaccination further solidify market leadership.
Asia Pacific Prophylactic Human Vaccine Market Analysis and Trends
Meanwhile, the Asia Pacific region exhibits the fastest growth with a CAGR exceeding 8%. Market expansion there benefits from rising healthcare expenditure, expanding immunization awareness, and supportive regulatory reforms. Countries such as India and China play vital roles due to their vast populations and increasing investments in vaccine manufacturing capabilities, including Serum Institute of India’s capacity ramp-up in 2025.
Prophylactic Human Vaccine Market Outlook for Key Countries
USA Prophylactic Human Vaccine Market Analysis and Trends
The USA's Prophylactic Human Vaccine market is a major contributor to global market revenue, bolstered by robust government funding and R&D investments, particularly in mRNA vaccine technologies. Initiatives such as the Vaccines for Children (VFC) program have expanded pediatric immunization coverage significantly, capturing substantial market share. Leading players like Pfizer, Moderna, and Merck maintain strong portfolios and pipeline innovations, supported by accelerated FDA approvals and collaborations, which drove a 14% revenue increase in 2024.
India Prophylactic Human Vaccine Market Analysis and Trends
India's market is rapidly evolving, supported by government immunization drives such as Mission Indradhanush and expanded vaccine production capacities. With key market companies like Serum Institute of India and Bharat Biotech leading manufacturing and export activities, the country has become a critical vaccine supplier globally. In 2025, indigenous vaccine development increased by 20%, reflecting growing local innovation. This market scenario places India at the forefront of the fastest-growing region with accelerated market penetration and revenue growth.
Analyst Opinion
The surge in demand for multi-valent vaccines is a crucial quantitative indicator supporting market expansion. For instance, in 2024, pentavalent vaccines recorded a 12% increase in production capacity worldwide, indicating manufacturers’ shift toward comprehensive immunization products. This production trend directly influences the market size and revenue streams by addressing broader disease prevention in a single dose.
Vaccine pricing remains a pivotal market driver, with cost-effective options emerging across developing regions. In 2025, price reductions up to 15% were noted in emerging economies due to government subsidies and local production efforts, enhancing accessibility and boosting vaccine uptake rates. This pricing dynamic underlies shifts in market share towards low-to-middle-income countries.
Demand-side indicators reveal diverse end-use industry applications, with pediatric immunization programs dominating usage patterns. Statistics from 2024 highlight that over 65% of total vaccine shipments were allocated to childhood vaccination campaigns, underscoring the segment’s influence on overall market revenue and growth strategies.
Supply chain resilience, including advancements in cold chain logistics, constitutes a critical micro-indicator. Recent expansions in cold storage capacity by 20% in 2024 across North America and Europe have minimized vaccine wastage, directly impacting market growth by maintaining consistent availability during peak demand periods.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: |
USD 72.8 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.8% | 2032 Value Projection: |
USD 115.3 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Sanofi Pasteur; Pfizer Inc.; GlaxoSmithKline plc; Merck & Co., Inc.; Bharat Biotech International Limited; Serum Institute of India Pvt. Ltd.; Johnson & Johnson; Novavax, Inc.; Sinovac Biotech Ltd.; BioNTech SE; AstraZeneca Plc. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Prophylactic Human Vaccine Market Growth Factors
The growing prevalence of infectious diseases globally continues to serve as a primary catalyst for market growth, exemplified by vaccination campaigns that increased overall market revenue by over 22% in 2024. Advancements in vaccine technology, particularly mRNA and recombinant platforms, optimize immunogenicity and development speed, propelling market dynamics positively. Furthermore, increased governmental investments in immunization infrastructure, as seen with the U.S. government’s CDC budget augmentation by 10% in 2025, facilitate broader vaccine coverage and sustained business growth. Finally, expanding immunization programs in emerging markets, fortified by international funding agencies’ support, directly contribute to the market’s increasing size and forecasted growth trajectory.
Prophylactic Human Vaccine Market Development
In June 2025, Cidara Therapeutics announced that it has received a BARDA (*Biomedical Advanced Research and Development Authority*) award to support the expanded manufacturing and clinical development of CD388, its non-vaccine, monoclonal-antibody-based influenza preventive therapeutic. The funding accelerates Cidara’s plans to scale production while advancing late-stage trials, highlighting public-private collaboration in bolstering pandemic preparedness and long-acting flu intervention strategies.
In 2025, American Immunization (Aim) announced that it is seeking regulatory approval for the world’s first serum-free human rabies vaccine, a next-generation biologic developed to enhance global rabies prevention through a more scalable, animal-component-free–free manufacturing approach.
Key Players
Leading Companies of the Market
Sanofi Pasteur
Pfizer Inc.
GlaxoSmithKline plc
Merck & Co., Inc.
Bharat Biotech International Limited
Serum Institute of India Pvt. Ltd.
Johnson & Johnson
Novavax, Inc.
Sinovac Biotech Ltd.
AstraZeneca Plc
Leading market players are actively adopting innovative growth strategies such as technology licensing, mergers & acquisitions, and collaborative R&D agreements. For instance, in 2024, Pfizer's collaboration with BioNTech to expand mRNA vaccine platforms significantly enhanced market penetration in adult immunization segments. Meanwhile, Serum Institute of India's aggressive capacity expansion initiatives in 2025 increased vaccine production volumes by nearly 18%, reinforcing its market revenue and share dominance within emerging regions.
Prophylactic Human Vaccine Market Future Outlook
The future of the prophylactic vaccine market lies in technological diversification and accessibility. mRNA and DNA-based platforms will continue to evolve for broad-spectrum protection and rapid pandemic response. The emergence of thermostable and needle-free formulations will improve global immunization rates, particularly in low-resource settings. Research into multivalent vaccines, universal influenza vaccines, and next-generation adjuvants will enhance immune response duration. Expanding vaccination programs for emerging diseases and improved manufacturing flexibility will sustain growth, ensuring prophylactic vaccines remain essential to global disease prevention efforts.
Prophylactic Human Vaccine Market Historical Analysis
The prophylactic vaccine market has its roots in the late 18th century, beginning with smallpox inoculation and expanding dramatically during the 20th century through widespread immunization programs. Initially focused on inactivated and live-attenuated vaccines, the field advanced with the introduction of conjugate, recombinant, and subunit vaccines for diseases like hepatitis B, HPV, and pneumococcal infections. The COVID-19 pandemic marked a turning point, accelerating the adoption of mRNA and viral vector platforms that revolutionized vaccine development speed and scalability. Global public health initiatives and government partnerships also boosted infrastructure for large-scale production and cold-chain logistics.
Sources
Primary Research Interviews:
Immunologists
Epidemiologists
Vaccine Researchers
Public Health Policy Experts
Databases:
WHO Global Health Observatory
CDC Immunization Data
GAVI Vaccine Information Portal
UNICEF Vaccine Dashboard
Magazines:
Vaccine Nation
Nature Biotechnology
Pharma Technology Focus
BioWorld Today
Journals:
Vaccine
The Lancet Infectious Diseases
Clinical Infectious Diseases
Nature Immunology
Newspapers:
The Guardian (Global Health)
The Hindu (Public Health)
The New York Times (Science)
Reuters Health
Associations:
World Health Organization (WHO)
Centers for Disease Control and Prevention (CDC)
GAVI Alliance
International Society for Vaccines (ISV)
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients