Pediatric Vaccines Market is estimated to be valued at USD 52,093.1 Mn in 2025 and is expected to reach USD 77,967.4 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 5.93% from 2025 to 2032.
Analysts’ Views on Global Pediatric Vaccines Market:
On October 6, 2021, The World Health Organization (WHO) announced that children in Sub-Saharan Africa and other areas with moderate to high P. falciparum malaria transmission are advised to receive the RTS, S/AS01 (RTS, S) malaria vaccine on regular basis. This advice was based on the outcomes of a trial programme that has been running in Ghana, Kenya, and Malawi, and has helped more than 900,000 kids in that regions since it began in 2019. Thus, awareness campaigns and vaccination campaigns can boost the market growth.
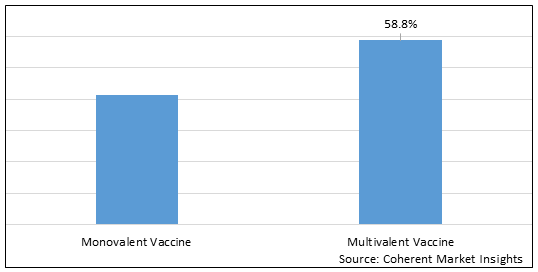
Figure 1. Global Pediatric Vaccines Market Share (%), By Type, 2025
To learn more about this report, Download Free Sample
Global Pediatric Vaccines Market- Drivers
Increasing regulatory approvals of vaccine candidates by key players
Increasing regulatory approvals of vaccine candidates by key market players is expected to boost the global pediatric vaccines market growth. For instance, in June 2022, Merck & Co., Inc., a U.S.-based multinational pharmaceutical company, announced that the U.S. Food and Drug Administration (FDA) has approved an expanded indication for VAXNEUVANCE (Pneumococcal 15-valent Conjugate Vaccine) for children of age 6 weeks to 17 years. VAXNEUVANCE is used for active immunization in children for the prevention of invasive disease caused by Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F in individuals of 6 weeks of age and older.
Pediatric Vaccines Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 52,093.1 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.93% | 2032 Value Projection: | USD 77,967.4 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
GlaxoSmithKline Plc., Merck & Co., Inc., Pfizer Inc., Sanofi S.A., Panacea Biotec, Zydus Cadila, Emergent BioSolutions Inc., Serum Institute of India Pvt. Ltd., Bharat Biotech, and Indian Immunologicals Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Increasing inorganic activities among government authorities
Increasing inorganic activities among government authorities is expected to drive the market growth over the forecast period. For instance, in August 2022, United Nations Programme on HIV/AIDS (UNAIDS), UNICEF, and the World Health Organization (WHO) entered into a global alliance to ensure that any child suffering with HIV (human immunodeficiency virus) will get proper treatment, and to prevent new infant HIV infections. The alliance has aimed to end AIDS in children by 2032.
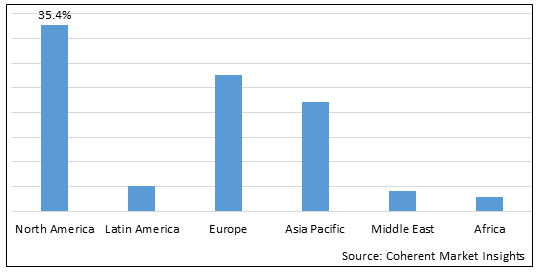
Figure 2. Global Pediatric Vaccines Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
Global Pediatric Vaccines Market- Regional Analysis
Among region, North America is expected to dominate the market over the forecast period, due to increasing vaccines approvals by regulatory authorities in region, and this is expected to drive the market growth over the forecast period. For instance, on June 18, 2025, the Centers for Disease Control and Prevention (CDC) approved COVID-19 vaccination for young children aged 6 months old that means around 20 million children in the U.S. are under 5 yearsof age, and are eligible for vaccination.
Europe region is expected to be the second largest region over the forecast period, owing to increasing product grants by regulatory authorities for usage of vaccine in pediatric population in the region, in order to expand their product portfolio is expected to drive the market growth over the forecast period. For instance, on February 24, 2022, European Medicines Agency’s human medicines committee (CHMP) granted an extension of indication for the COVID-19 vaccine Spikevax for usage in children of age 6 to 11 years. The vaccine is developed by Moderna, is a pharmaceutical and biotechnology company and is already approved for usage in adults and children age 12 years and above.
Global Pediatric Vaccines Market– Impact of Coronavirus (COVID-19) Pandemic
Coronavirus disease 2019 (COVID-19) is a highly contagious infectious disease that is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). According to the World Health Organization (WHO), 753,651,712 people were affected globally till January 30, 2023.
However, the COVID-19 pandemic had a positive impact on the global pediatric vaccines market, due to increasing vaccination campaign during COVID-19 pandemic. For instance, on August 11, 2022, The World Health Organization (WHO) along with the Strategic Advisory Group of Experts (SAGE) on Immunization and its COVID-19 Vaccines Working Group, reviewed the emerging evidence on need for and timing of vaccinating children and adolescents with the currently available COVID-19 vaccines that has received Emergency Use Listing (EUL). SAGE is continuously reviewing the Research studies and has reached out to vaccine manufacturers, the research community, and Member States to obtain complete and recent data. This interim statement was developed with additional support from the Strategic and Technical Advisory Group of Experts (STAGE) on maternal, newborn, child, and adolescent health and nutrition.
Global Pediatric Vaccines Market Segmentation:
The global pediatric vaccines market report is segmented into Vaccine Type, Technology, Type, End User, Distribution Channel and Region
Based on Vaccine Type, global pediatric vaccines market is segmented into Pneumococcal Vaccine, DTP Vaccine, Rotavirus Vaccine, MMR Vaccine, Polio Vaccine, Influenza Vaccine, Hepatitis B Vaccine, Meningococcal Vaccine, Hib Vaccine, and Varicella Vaccine. Influenza vaccine segment is expected to dominate the market over the forecast period, due to increasing Biologics License Application (BLA) for vaccines by regulatory authorities. For instance, on January 5, 2025, The U.S. Food and Drug Administration (FDA) Center for Drug Evaluation and Research (CDER) accepted the Biologics License Application (BLA) for nirsevimab for prevention of respiratory syncytial virus (RSV) lower respiratory tract disease in newborns and infants entering, and for children up to 24 months of age who can be vulnerable to severe RSV disease through their second RSV season.
Based on Technology, the market is segmented into Live or Attenuated Vaccine, Inactivated or Killed Vaccine, Toxoid Vaccine, Conjugate Vaccine, Subunit Vaccine, Recombinant Vaccine. Out of which, live or attenuated vaccine segment is expected to dominate the market over the forecast period, due to increasing launch of vaccines by key market players. For instance, in January 2020, Serum Institute of India Pvt. Ltd., a biotechnology company, launched a new variant of its World Health Organization (WHO) prequalified rotavirus vaccine Rotasiil named Rotasiil– Liquid, a live attenuated oral vaccine. ROTASIIL-Liquid is administered as a 3-dose regimen, 4 weeks apart, and children of age 6 weeks. The three dose regimen should be completed by one year of age. ROTASIIL-Liquid can be co-administered with other routine childhood immunizations (i.e., Diphtheria, Tetanus and Pertussis [DTwP], Hepatitis B vaccine, H. influenza type b (Hib) vaccine, inactivated polio vaccine (IPV), and Oral Polio Vaccine [OPV]).
Based on Type, the market is segmented into Monovalent Vaccine and Multivalent Vaccine. Out of which, monovalent vaccine segment is expected to dominate the global pediatric vaccines market during the forecast period, due to increasing vaccines approvals by regulatory authorities in the market. For instance, in 2021, GlaxoSmithKline PLC, a pharmaceutical and Biotechnology Company, and CureVac N.V., a biopharmaceutical company, announced a new US$ 152.6 million collaboration, in order to jointly develop next generation mRNA vaccines for multivalent COVID-19.
Based on Distribution Channel, global pediatric vaccines market is segmented into Government and Private. Of which, government segment is expected to dominate the market over the forecast period, owing to increasing recommendation for vaccination campaigns by international healthcare organization in Pediatric Clinics. For instance, on January 02, 2022, the Centers for Disease Control and Prevention (CDC) recommended that all children of age 6 months to 5 years of age should receive COVID-19 vaccine. Distribution of pediatric vaccinations for younger children has started across the U.S. and will be available at thousands of pediatric practices, pharmacies, Federally Qualified Health Centers, local health departments, and clinics.
Based on Region, the pediatric vaccines market is segmented into North America, Latin America, Europe, Middle East, Asia Pacific, and Africa. Of which, North America segment is expected to dominate the market over the forecast period, due to increasing inorganic growth strategies such as collaboration by key market players in the region.
Among all segmentation, Vaccine Type segment is expected to drive the growth of market owing to the due to increasing prevalence of bacterial infection, and this is expected to drive the market growth over the forecast period. For instance, according to the data provided by the Centers for Disease Control and Prevention (CDC), in March 2022, typhoid fever affects over 10 million people annually, and causes over 116,000 deaths per year, mostly among children, globally.
Global Pediatric Vaccines Market- Cross Sectional Analysis:
In Technology segment, toxoid vaccine segment held a dominant position in Africa region, due to increasing inorganic activities such as partnerships among market players. For instance, in March 2021, Cipla, a pharmaceutical company, entered into a partnership with the Serum Institute of India, a biotechnology company, and launched a new tetanus toxoid vaccine that can be used for prevention of tetanus infection in infants, children, and adults in South Africa.
Global Pediatric Vaccines Market: Key Developments
On August 5, 2022, The Republic of the Congo launched a preventive mass vaccination campaign with aim to vaccinate more than 93% of the population against yellow fever. This campaign will target over 4 million people in 11 out of the 12 healthcare departments across the Republic of the Congo The country has set a goal of achieving more than 95% of national vaccine coverage. The campaign is supported by Gavi, a Vaccine Alliance, United Nations Children's Fund (UNICEF), and the World Health Organization (WHO) and partners.
According to an article published by the Lancet Infectious Diseases Journal, a peer-reviewed scientific journal, in October 2021, a clinical study was conducted in Ho Chi Minh City, Vietnam, to determine the immunogenicity of alternative ten-valent pneumococcal conjugate vaccine (PCV 10) schedules in infants of age two months. The results of this study revealed that three-dose primary vaccination series was more efficient in providing protection against pneumococcal bacteria as compared to two-dose primary vaccination series.
Global Pediatric Vaccines Market: Key Trends
For instance, on June 22, 2022, Contra Costa, U.S., announced that it will run vaccination clinics in order to provide safe and effective COVID vaccines to children age 6 months to 4 years old. The U.S. Food and Drug Administration (FDA), the Centers for Disease Control and Prevention (CDC), and the Western States Scientific Safety Review Workgroup, authorized pediatric versions of Pfizer's and Moderna's vaccines for above age group. Earlier, only children of age more than 5 years and adults were eligible to receive COVID vaccinations.
Global Pediatric Vaccines Market: Restraint
Adverse effects after vaccination
The major factors that can hamper growth of the global pediatric vaccines market over the forecast period include serious adverse events after COVID-19 boosters in young kids. For instance, on August 18, 2022, The University of Minnesota, U.S., published the data collected from two vaccine safety surveillance programs in the first 10 weeks of administration of third doses of the Pfizer/BioNTech COVID-19 vaccine to children aged 5 to 11 years in the U.S., and it had serious adverse effects such as injection-site pain, fatigue, and headache.
Therefore, study of adverse effects occurred during treatment is required for treatment improvisation which expected to drive the growth of market in near future
Global Pediatric Vaccines Market: Key Players
Major players operating in the global pediatric vaccines market include GlaxoSmithKline Plc., Merck & Co., Inc., Pfizer Inc., Sanofi S.A., Panacea Biotec, Zydus Cadila, Emergent BioSolutions Inc., Serum Institute of India Pvt. Ltd., Bharat Biotech, and Indian Immunologicals Ltd.
*Definition: Vaccination is administration of a vaccine into human body, in order to help immune system in developing immunity against a disease. There are two age groups based on which vaccines are provided: the pediatric age group, and the adult age group. The pediatric age group ranges from birth to 18 years.
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients