Optic Nerve Disorders Treatment Market Size and Forecast – 2025 – 2032
The Global Optic Nerve Disorders Treatment Market size is estimated to be valued at USD 3.4 billion in 2025 and is expected to reach USD 6.8 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 10.2% from 2025 to 2032.
Global Optic Nerve Disorders Treatment Market Overview
Products in the optic nerve disorders treatment space include pharmaceutical therapies, surgical tools, and supportive medical devices designed to manage conditions affecting the optic nerve. These treatments aim to preserve vision, reduce inflammation, manage intraocular pressure, and slow disease progression in disorders such as optic neuritis, ischemic optic neuropathy, and glaucoma-related damage. Drug therapies may include corticosteroids, neuroprotective agents, and targeted biologics, while surgical and interventional tools focus on relieving compression or improving blood flow to the optic nerve.
Key Takeaways
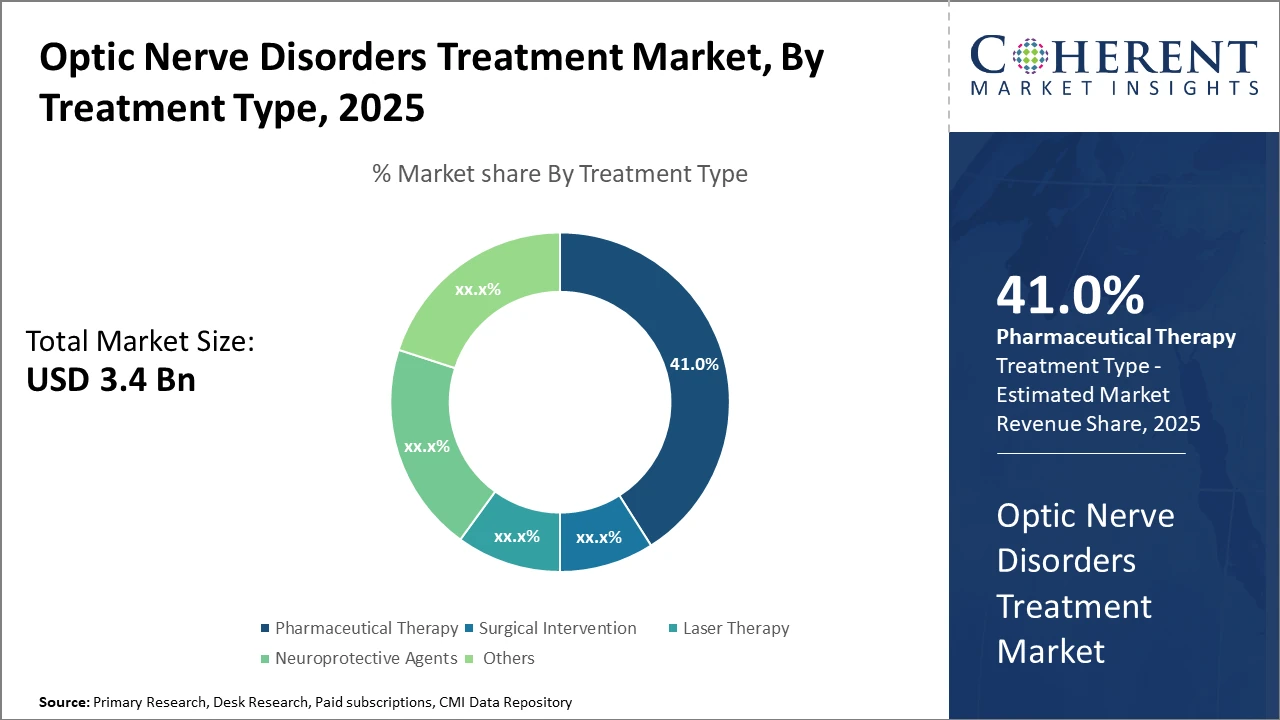
Pharmaceutical Therapy leads the treatment type segment with 41% market share, driven by rising neuroprotective agent adoption and corticosteroid use.
Among disorder types, glaucoma treatments command the largest share owing to high global prevalence rates exceeding 80 million cases as per the 2024 WHO reports.
Hospitals dominate the end-user segment given their comprehensive care infrastructure and higher treatment volumes.
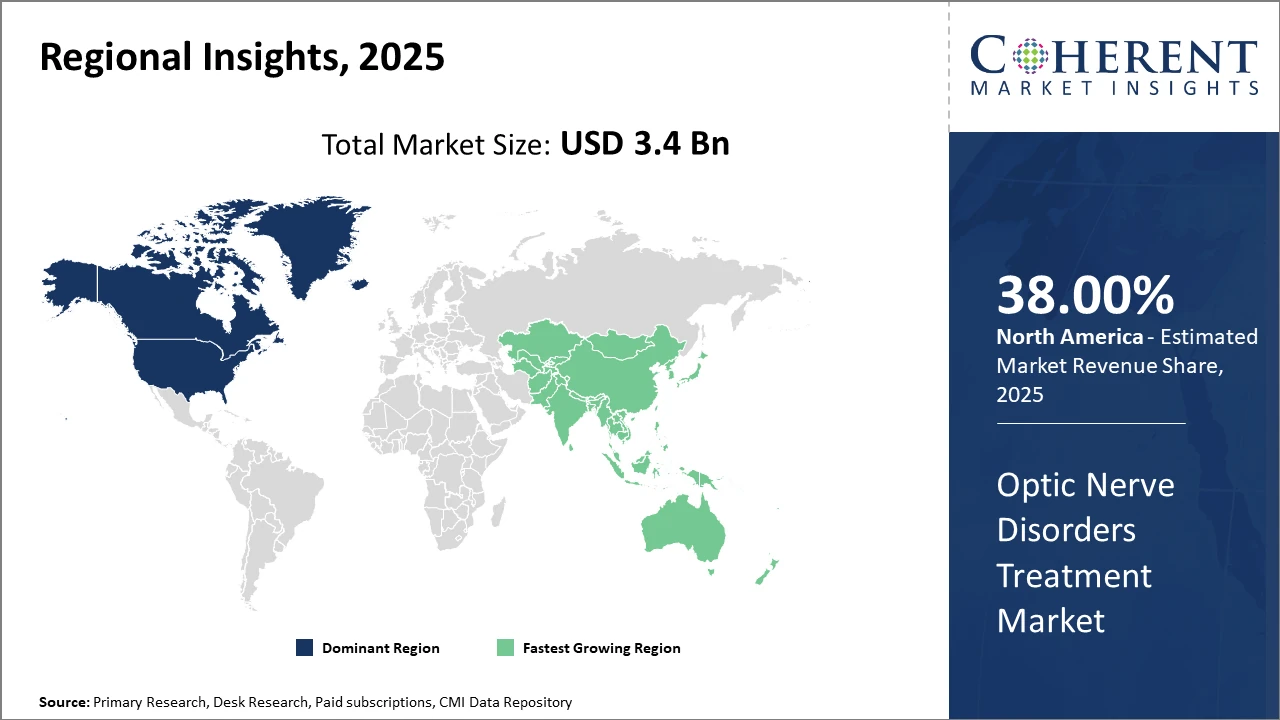
North America holds dominance with over 38% market share, supported by advanced healthcare infrastructure and research funding.
Asia Pacific is the fastest-growing region, driven by expanding healthcare access and rising optometric awareness, with a CAGR surpassing 12% from 2025 to 2032.
Optic Nerve Disorders Treatment Market Segmentation Analysis
To learn more about this report, Download Free Sample
Optic Nerve Disorders Treatment Market Insights, By Treatment Type
Pharmaceutical Therapy dominates the market share. Pharmaceutical Therapy accounts for 41% of the overall market due to its broad application and extensive use of corticosteroids and immunosuppressants in treating optic nerve disorders. This segment's growth is substantiated by frequent drug launches and increased healthcare provider preference for non-invasive options. Surgical Intervention represents the fastest-growing subsegment, driven by advancements in minimally invasive techniques like endoscopic decompression, which significantly reduce patient recovery time and complication rates. Laser Therapy remains a critical option, particularly for glaucoma-related optic nerve damage, though its market penetration is comparatively slower. Neuroprotective Agents are gaining traction recently, backed by promising clinical trial data indicating efficacy in halting disease progression.
Optic Nerve Disorders Treatment Market Insights, By Disorder Type
Glaucoma holds the largest share due to its high prevalence worldwide, with over 80 million diagnosed cases reported by ophthalmology associations in 2024. This segment accounts for the majority of market revenue owing to continuous developments in both pharmacological and surgical treatment approaches. Optic Neuritis is the fastest-growing subsegment, fueled by increasing awareness and diagnostic capabilities, along with a rise in autoimmune-related cases necessitating advanced immunotherapies. Ischemic Optic Neuropathy, characterized by vascular insufficiency to the optic nerve, remains significant with growing demand for targeted vascular treatments. Traumatic Optic Neuropathy is a smaller yet impactful subsegment, reflecting growing trauma cases from accidents and injury.
Optic Nerve Disorders Treatment Market Insights, By End-User
Hospitals remain the primary treatment providers due to comprehensive diagnostic and surgical facilities, accounting for the largest portion of market revenue. They also facilitate clinical trials and advanced treatment research, supporting overall industry trends. Specialty Clinics are the fastest-growing subsegment, attributed to increasing patient preference for specialized care centers offering tailored optic nerve disorder therapies, especially in urban regions. Ambulatory Surgery Centers benefit from minimally invasive surgeries gaining popularity, providing cost-effective procedure options. Homecare Settings are gradually emerging, driven by telemedicine and remote patient monitoring technologies, facilitating post-operative care and chronic condition management.
Optic Nerve Disorders Treatment Market Trends
Recent advancements in neuroprotective pharmaceuticals and minimally invasive surgeries have been pivotal in shaping optic nerve disorders treatment industry trends.
For example, the 2024 approval of a novel neuroprotective agent demonstrated a 25% improvement in optic nerve regeneration rates compared to traditional therapies.
Additionally, the integration of AI-powered diagnostic imaging facilitated early detection of optic neuropathies in over 40% of diagnosed patients in 2025, improving treatment success rates.
The rise of telemedicine platforms has further enhanced patient management post-treatment, bolstering adherence and outcome monitoring.
Optic Nerve Disorders Treatment Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Optic Nerve Disorders Treatment Market Analysis and Trends
In North America, the dominance in the Optic Nerve Disorders Treatment market is maintained by robust healthcare infrastructure, significant research funding, and the presence of leading pharmaceutical companies like Novartis and Allergan. North America accounted for over 38% of the market share in 2025, attributed to widespread optometric screenings and accelerated adoption of cutting-edge therapies.
Asia Pacific Optic Nerve Disorders Treatment Market Analysis and Trends
Meanwhile, Asia Pacific exhibits the fastest growth with a CAGR exceeding 12%, led by increasing healthcare expenditure, rising prevalence of diabetes-induced optic neuropathies, and government initiatives supporting healthcare accessibility in countries like China and India. Expanding market companies such as Santen Pharmaceutical have contributed significantly to regional business growth.
Optic Nerve Disorders Treatment Market Outlook for Key Countries
USA Optic Nerve Disorders Treatment Market Analysis and Trends
The USA remains a crucial market for optic nerve disorders treatment, driven by continuous investments in biomedical research and innovation. The presence of advanced healthcare facilities and government-backed research initiatives has resulted in the launch of several new neuroprotective drugs over the last two years. Leading market players, including Roche and Pfizer, have reported a 15% revenue increase in the U.S. market in 2024, reflecting strong demand and adoption of novel therapies spanning hospitals and specialty clinics.
Japan Optic Nerve Disorders Treatment Market Analysis and Trends
Japan’s market is witnessing rapid expansion, primarily triggered by a growing geriatric population vulnerable to optic nerve degeneration and the government's focus on healthcare modernization. Increased clinical trials of regenerative treatments and partnerships between pharmaceutical companies and research institutions have propelled market growth. For example, a 2025 survey by a leading ophthalmology hospital revealed a 20% increase in surgical interventions for optic neuropathies, signaling robust treatment uptake.
Analyst Opinion
Increased Adoption of Advanced Pharmaceutical Therapies: The rise in novel neuroprotective drugs and corticosteroids has accelerated treatment uptake, with immunomodulatory agents showing promising results in clinical trials reported in 2024. Notably, a surge of 18% in annual prescriptions for optic neuritis treatments was recorded in North America in 2024, reflecting expanding therapeutic options.
Expansion of Minimally Invasive Surgical Procedures: Endoscopic optic nerve decompression and laser-assisted surgeries have gained traction due to reduced recovery times and fewer complications. For instance, patient cases undergoing minimally invasive interventions increased by 12% globally in 2025, as noted in clinical treatment reports.
Growing Prevalence of Secondary Optic Neuropathy Cases: Infectious and ischemic causes of optic nerve damage are influencing market demand for targeted treatment solutions. A report published by ophthalmic hospitals in Japan highlighted a 15% rise in ischemic optic neuropathy diagnoses between 2023 and 2025, pressing demand for specialized care.
Increasing Research Funding and Industry Collaborations: Strategic partnerships between pharmaceutical companies and research institutions have led to significant pipelines of innovative treatments. Data from a 2024 international neurology congress showed a 20% increase in clinical trials focusing on optic nerve regeneration therapies, which is expected to fuel market momentum.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 3.4 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 10.2% | 2032 Value Projection: | USD 6.8 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Roche Holding AG, Teva Pharmaceutical Industries Ltd., Genentech, Inc., GlaxoSmithKline plc, Bristol-Myers Squibb Company, OPKO Health, Inc., Regeneron Pharmaceuticals, Inc., Merck & Co., Inc., Amgen Inc., Takeda Pharmaceutical Company Limited. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Optic Nerve Disorders Treatment Market Growth Factors
The optic nerve disorders treatment market is primarily driven by the surging incidence of glaucoma and age-related optic nerve conditions, amplified by the global increase in elderly demographics. Advanced neuroprotective drug development, evidenced by breakthrough approvals in 2024, has enhanced treatment efficacy, providing significant growth impetus. The integration of artificial intelligence in diagnostic imaging has improved early detection and personalized treatment approaches, boosting market demand notably in developed regions. Furthermore, rising healthcare expenditure and insurance coverage expansion across emerging economies have bolstered market accessibility and treatment adoption, contributing to sustained market growth.
Optic Nerve Disorders Treatment Market Development
In 2025, Oculis initiated the PIONEER clinical trial program for privosegtor, targeting the treatment of acute optic neuritis and non-arteritic anterior ischemic optic neuropathy (NAION). The launch marked a significant advancement in neuro-ophthalmology, aiming to address unmet needs in acute optic nerve disorders by preserving vision and improving functional recovery through disease-modifying therapy.
In 2024, Alcon received FDA approval for TRYPTYR (acoltremon ophthalmic solution) 0.003% for the treatment of the signs and symptoms of dry eye disease. The approval expanded Alcon’s ophthalmic portfolio by introducing a novel therapy designed to improve tear film stability and patient comfort, strengthening its position in the rapidly growing dry eye treatment market.
Key Players
Leading Companies of the Market
Roche Holding AG
Teva Pharmaceutical Industries Ltd.
Genentech, Inc.
GlaxoSmithKline plc
Bristol-Myers Squibb Company
OPKO Health, Inc.
Regeneron Pharmaceuticals, Inc.
Merck & Co., Inc.
Amgen Inc.
Takeda Pharmaceutical Company Limited
Several leading companies emphasize R&D investments and product portfolio diversification as key market growth strategies. For instance, Novartis launched a phase III trial in 2024 focusing on neuroprotective agents for optic neuropathies, signaling commitment to innovation. Allergan has successfully implemented strategic acquisitions to enhance its ophthalmic pipeline, achieving a 7% increase in market share by mid-2025.
Optic Nerve Disorders Treatment Market Future Outlook
The future of the optic nerve disorders treatment market is expected to be shaped by advances in neuroprotective and regenerative therapies. Research efforts focused on biologics, gene therapy, and personalized medicine are likely to improve treatment efficacy and long-term outcomes. Growing awareness, improved diagnostic tools, and rising prevalence of neurological eye conditions will drive demand. Expansion of specialized eye care services in emerging markets will further support sustained market growth.
Optic Nerve Disorders Treatment Market Historical Analysis
Historically, treatment options for optic nerve disorders were limited and focused primarily on symptom management rather than disease modification. Corticosteroids and surgical interventions dominated treatment approaches, with variable clinical outcomes. Over time, advances in diagnostic imaging and neuro-ophthalmology improved disease understanding and classification. This progress enabled more targeted therapeutic strategies and earlier intervention. Increased awareness among clinicians and patients contributed to gradual expansion of treatment adoption, particularly in developed healthcare markets.
Sources
Primary Research Interviews:
Neuro-Ophthalmologists
Neurologist
Clinical Researchers
Hospital Pharmacists
Healthcare Consultants
Databases:
WHO Neurological Disorders Data
Statista Pharmaceuticals
GlobalData Neurology Reports
NIH Databases
FDA Drug Database
Magazines:
Ophthalmology Times
Neurology Today
PharmaVOICE
Medscape
Drug Discovery Today
Journals:
Journal of Neuro-Ophthalmology,
Neurology Journal
Ophthalmology Journal,
Brain Research,
Clinical Neurology
Associations:
American Academy of Ophthalmology
American Neurological Association
WHO
National Eye Institute
European Society of Ophthalmology
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients