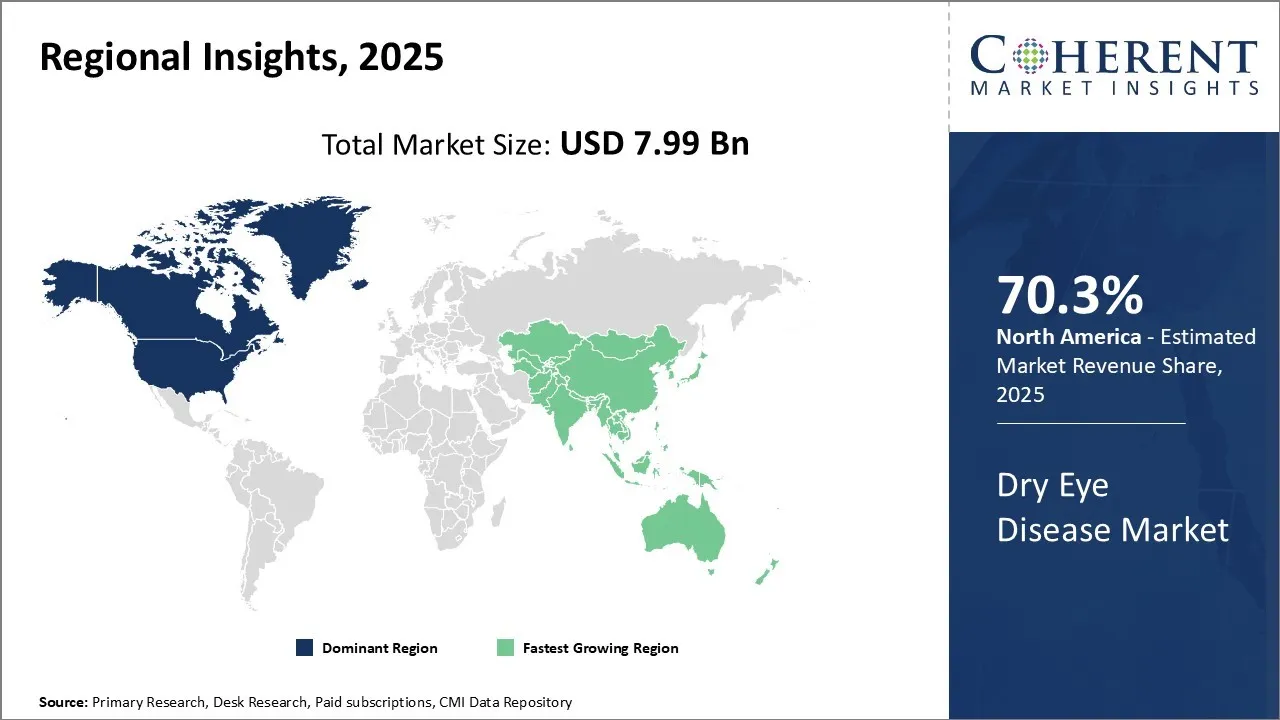
The dry eye disease market is estimated to be valued at USD 7.99 Bn in 2025 and is expected to reach USD 14.62 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 9.0% from 2025 to 2032.
To learn more about this report, Download Free Sample
The dry eye disease market is expected to witness steady growth during the forecast period. This growth is attributed to rising geriatric population worldwide who are more prone to developing dry eye disease. Additionally, the increasing prevalence of chronic diseases, such as diabetes, which can cause tear deficiency is also contributing to the growth of the market.
With growing awareness about disease diagnosis and treatment options, there is higher demand for prescription and over-the-counter medications to manage the symptoms of dry eyes. Furthermore, technological advancements in incorporating artificial tears, punctum plugs, and other medical devices for dry eye treatment is expected to provide new opportunities for market players over the next few years.
|
Current Event |
Description and its Impact |
|
Technological Advancements in Ophthalmology |
|
|
Regulatory Environment Evolution |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
|
Region |
Prevalence / Key Data |
Notes |
|
North America |
~16 million diagnosed in the U.S.; undiagnosed cases may reach 30–49 million |
Highest prevalence due to aging, screen use, and strong diagnostic infrastructure |
|
Europe |
Estimated 10–30% of adults affected, with higher rates in older populations |
Aging demographics and advanced healthcare systems sustain steady prevalence |
|
Asia-Pacific |
Prevalence ranges 20–30% in countries like China, India, Japan, South Korea |
Fastest growth region; driven by pollution, digital lifestyles, and urbanization |
|
Latin America |
~15–20% prevalence in countries like Brazil and Argentina |
Rising demand with expanding healthcare access |
|
Middle East & Africa |
~10–20% prevalence, with hotspots in Turkey, Israel, GCC countries, South Africa |
Increasing awareness and ophthalmology care availability |
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The product type, the cyclosporine segment is estimated to hold 37.8% share of the market in 2025. Ophthalmic cyclosporine is used to increase tear production in people suffering from dry eye disease. Cyclosporine is an immunomodulator and functions by decreasing swelling in the eye to allow tear production. Cyclosporine is available in solution (liquid) and emulsion forms to treat dry eye syndrome. Increasing product launches by key players in the market is expected to drive the segment growth over the forecast period.
For instance, in march 2025, Harrow started the VEVYE® Access for All program, which makes VEVYE (cyclosporine ophthalmic solution 0.1%) more widely available across the country for people with dry eye disease. The goal of the initiative is to make treatment easier to get by offering low prices. Patients who qualify can pay as little as $59 per bottle or even absolutely nothing if they have insurance. PhilRx will also deliver the medication for free.
The formulation type, the liquid subsegment is expected to have 57.3% of the market share in 2025. Liquid formulations are most widely utilized to treat dry eye disease symptoms attributed to its ease of application. There are several liquid formulations available in the market such as Restasis, Cequa, Xiidra, and Eysuvis. Most of the formulations contain cyclosporine as an active ingredient (Restasis, Cequa) while some have lifitegrast (Xiidra), and corticosteroid (Eysuvis) as an active ingredient. Rise in the number of clinical trials being undertaken by several clinical stage companies is anticipated to drive the liquid segment growth.
For instance, in May 2025, Alcon reported that the FDA had approved TRYPTYR (acoltremon ophthalmic solution 0.003%), a new liquid eye drop that can help with the signs and symptoms of dry eye disease. The company plans to launch TRYPTYR in the U.S. soon and then make it available in other markets, which will strengthen its portfolio of eye care products.
The distribution channel, the retail pharmacies subsegment is expected to have 50.1% of the market share in 2025. Being located within communities, retail pharmacies offer patients easy geographic access without needing an appointment or visit to an ophthalmologist. This proximity and walk-in availability make retail pharmacies very convenient for treatment and refill requirements. In addition, major retail pharmacy chains have dedicated ocular departments staffed with optometrists and well-trained pharmacists who are able to appropriately counsel patients about their dry eye conditions and medications. Increasing expansion and launches of retail drug chains is expected to drive the segment growth over the forecast period.
For instance, in February 2023, Aster Pharmacy, a division of Aster DM Healthcare, a modern-day healthcare ed-tech company opened its 250th pharmacy in Elamakkara, India. Aster Pharmacy which is a subsidiary of Alfaone Retail Pharmacies, a private limited company, announced the inauguration of its 200th location in September 2022. Aster Pharmacy has 507 pharmacies across the U.A.E., Saudi Arabia, Oman, Qatar, Bahrain, Jordan, India, and Bangladesh.
To learn more about this report, Download Free Sample
North America remains the dominant region in the global dry eye disease market and is anticipated to hold 70.3% of the market share in 2025. With high consumer awareness regarding eye health issues, many patients in the U.S. and Canada seek early diagnosis and treatment for dry eye symptoms. This has created a strong demand for various dry eye drugs and devices in the healthcare setting. Increasing number of research studies coupled with growing prevalence of dry eye diseases are the major factors anticipated to drive the growth of the market in this region over the forecasted period.
For instance, in May 2025, the FDA gave Grifols the permission to start a Phase II clinical trial of GRF312 ophthalmic solution, an experimental immunoglobulin eye drops for dry eye disease. The study in the U.S. will examine at the effectiveness, safety, and tolerability in 100 patients. It marks an important development toward moving new therapies beyond current ones like cyclosporine and lifitegrast.
The Asia Pacific region, especially China and India, is emerging as the fastest growing market for dry eye disease. Growing industrialization and extensive time spent in front of digital screens have significantly contributed to increased screen time and dry eye cases in Asian countries in recent years. Moreover, the geriatric population in major Asian economies is rising rapidly, adding to the risk of age-related dry eye conditions. Regulatory approvals for clinical trials of novel drugs for the treatment of dry eye disease are anticipated to drive the growth of the market in the Asia Pacific region over the analysis period.
For instance, in July 2025, The National Medical Products Administration (NMPA) of China approved Hengrui Pharma's Heng Qin (Perfluorohexyloctane Eye Drops). This is the first treatment in the country for dry eye disease linked to Meibomian gland dysfunction (MGD). The drops, which are based on EyeSol® water-free technology, reduce the evaporation of tears and speed up the healing of the cornea after successful Phase 3 trials. It is expected that the product will be available for sale in China soon.
The U.S. dry eye disease market is highly demanding due to its large patient base of more than 20 million Americans with dry eye disease. There are more than 20 million Americans with dry eye disease, and the market is very competitive because of this. The disease is caused by aging populations, high screen time, and lifestyle factors. The U.S. is the largest market in the world for therapies like Restasis and Xiidra due to individuals are very aware of them, the healthcare system is very advanced, as well as are many retail pharmacies that sell them.
For instance, in May 2025, Aldeyra Therapeutics confirmed that its experimental drug Reproxalap reached the main goal in a Phase 3 clinical trial for dry eye disease. The results showed that the drug could be a new treatment option as it made patients' symptoms much better. Aldeyra plans to move forward with regulatory submissions, which is an enormous step forward in the development of eye drugs.
China's dry eye disease market is growing strongly mainly because more individuals are getting it because of things like living in cities, using digital screens excessively, exposure to pollution, and growing older. Demand is rising awareness, diagnoses are getting better, and regulatory approvals including Heng Qin® and Diquafosol Sodium Eye Drops are coming through. China is one of the fastest-growing dry eye markets in the world considering it has a strong healthcare system and more people need care.
For instance, in May 2025, The National Medical Products Administration (NMPA) in China approved Uni-Bio Science Group's Diquafosol Sodium Eye Drops, which could assist people with dry eye syndrome. The approval is an important advancement forward in the field of ophthalmology. It gives patients in China more treatment options and makes Uni-Bio's presence stronger in the growing dry eye disease market.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 7.99 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.0% | 2032 Value Projection: | USD 14.62 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
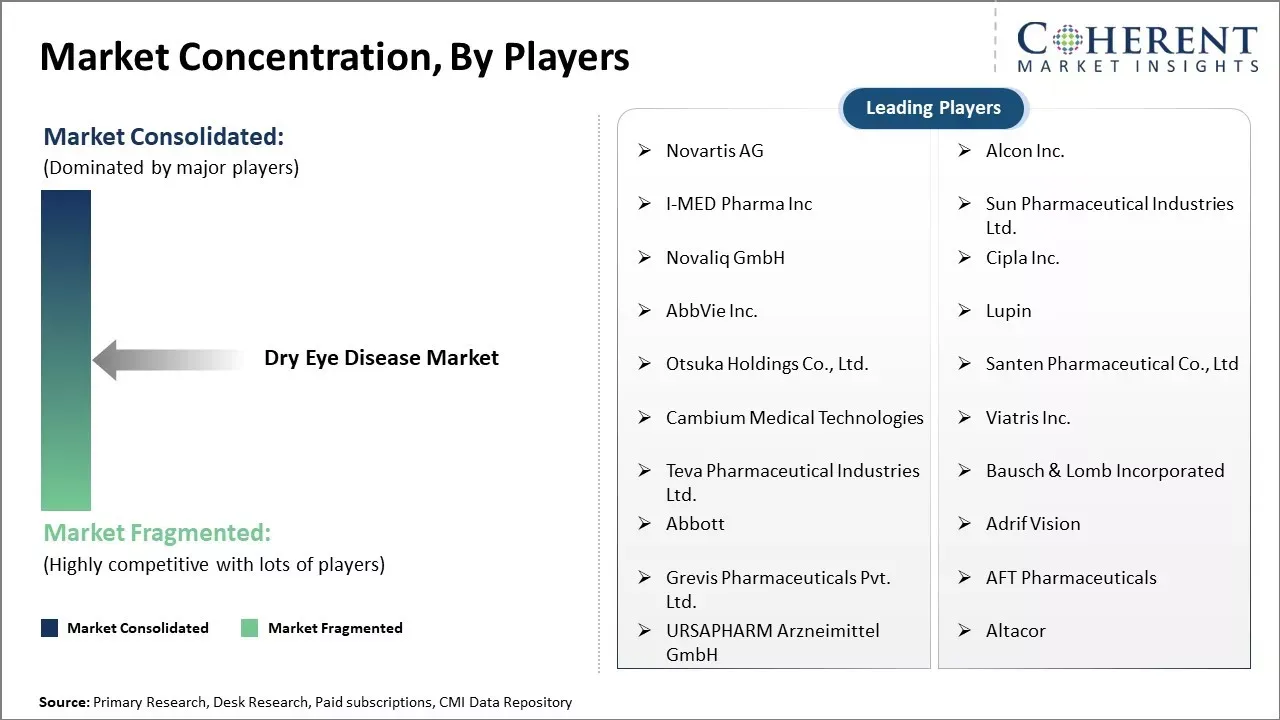
Novartis AG, Alcon Inc., I-MED Pharma Inc, Sun Pharmaceutical Industries Ltd., Novaliq GmbH, Cipla Inc., AbbVie Inc., Lupin, Otsuka Holdings Co., Ltd., Santen Pharmaceutical Co., Ltd, Cambium Medical Technologies, Viatris Inc., Teva Pharmaceutical Industries Ltd., Bausch & Lomb Incorporated, Abbott, Adrif Vision, Grevis Pharmaceuticals Pvt. Ltd., AFT Pharmaceuticals, URSAPHARM Arzneimittel GmbH, and Altacor |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The increasing number of product approvals and launches for dry eye disease treatment over the past couple of years has provided a significant boost to the dry eye disease market. With the disease affecting a substantial portion of the global population, especially with rising screen time and digital device usage, the demand for effective treatment options has grown exponentially.
For instance, in February 2023, Aldeyra Therapeutics, Inc., a company discovering and developing innovative therapies designed to treat immune-mediated diseases, announced that the U.S. Food and Drug Administration (FDA) had accepted the New Drug Application (NDA) for topical ocular reproxalap, a first-in-class investigational new drug candidate, for the treatment of the signs and symptoms of dry eye disease.
People who frequently use computer or screens are also at high risk of developing dry eyes, as using screens for a long period can lead to reduced blinking and tear production. Digital device usage has increased significantly in recent years among all age groups, due to extensive use for social and professional purposes. Blue light emitted by digital screens is associated with symptoms of dry eye syndrome. Exposure to blue light (range of 400–500 nm) can be harmful to the retina. Longer duration and less intense light exposure can also induce photochemical damage in the eyes. Furthermore, lack of sleep and excessive stress can play a crucial role in the onset of dry eye syndrome due to oily tear gland dysfunction.
According to the data provided by SleepFoundation.org, a foundation providing information about sleep and promoting overall wellness, in May 2023, more than one-third of U.S. adults sleep less than seven hours per night, on average. According to the same source, 50 million to 70 million people in the U.S. have ongoing sleep disorders.
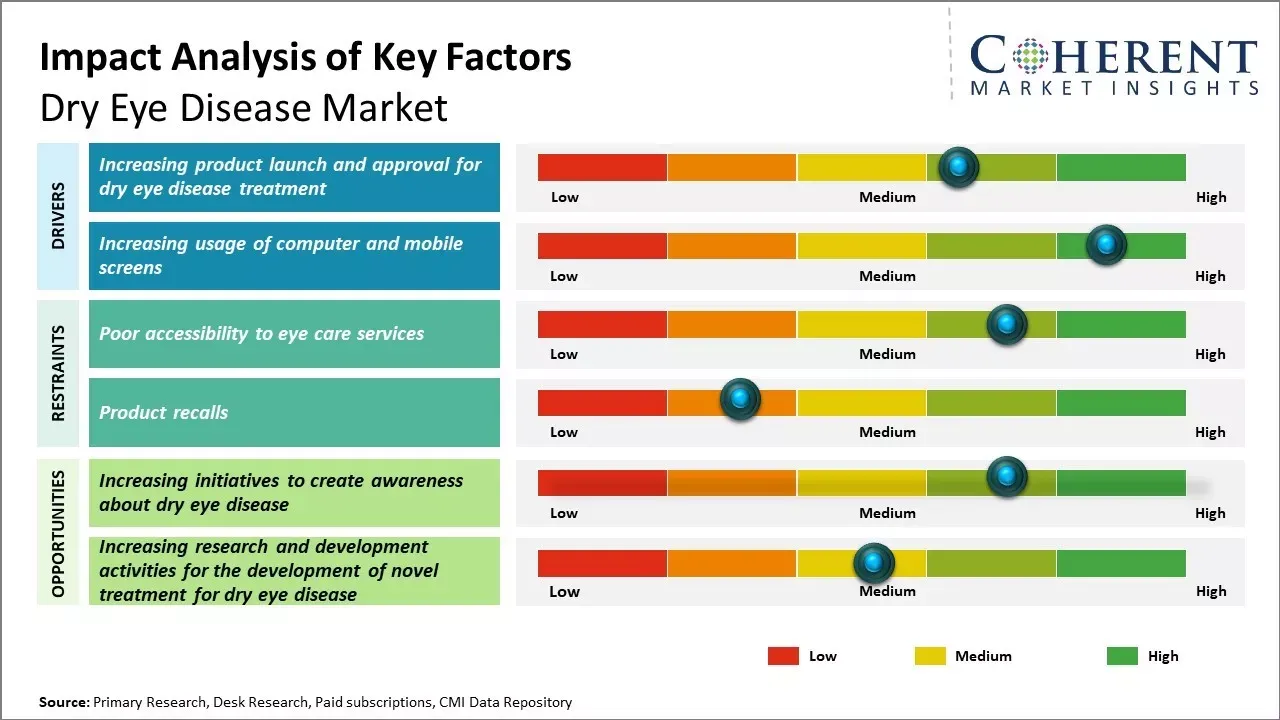
Increasing initiatives to create awareness about dry eye disease are boosting diagnosis rates and treatment adoption globally. Educational campaigns, patient outreach, and professional training enhance recognition of symptoms, driving demand for advanced therapies. These efforts significantly contribute to the growth trajectory of the dry eye disease market forecast, strengthening future market expansion.
To learn more about this report, Download Free Sample
The Dry Eye Disease market value holds an extensive amount of potential to grow as there are so countless individuals who have it. The global prevalence of symptoms is usually between 9% and 12%, but it is much higher in older adults and women. Regional data show that the rates are different from one study to the next, with some population studies showing rates above 30%. This shows that the diagnosis is rarely consistent and that there is a large group of people who have not been diagnosed. This keeps the demand for over-the-counter lubricants, prescription anti-inflammatories, secretagogues, and device-based therapies high.
Surveys show that more than 70% of people who get some kind of DED care use artificial tears, making them the most common type of treatment. However, lubricants only provide short-term relief, which is speeding up the use of prescription therapies and in-office procedures that target meibomian gland dysfunction, which is one of the most common causes of evaporative dry eye. Recent regulatory approvals for novel tear-stimulation and anti-inflammatory agents are expanding the therapeutic mix and encouraging transitions from OTC to prescription care.
Economic burden studies show meaningful direct and indirect costs, with annual per-patient expenses nearing USD 800 when including medical visits and symptomatic management. As populations age and digital-device exposure intensifies, opportunities lie in improved diagnostics, adherence-focused drug-delivery systems, and evidence-backed disease-modifying therapies capable of reducing chronic symptom cycles and enhancing patient retention within the treatment pathway.
Definition: The dry eye disease market involves products that aid in diagnosing and treating dry eye disease, a condition where a person does not produce enough tears or their tears do not have the correct properties to lubricate and nourish the eyes. Products in this market include artificial tear eye drops that lubricate dry eyes, cyclosporine eye drops that reduce inflammation, tear duct plugs that prevent tear drainage, ointments and gels to lubricate eyes overnight, devices like humidifiers and heat masks that add moisture to the eyes.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients