Needle-Free Injection System Market Size and Forecast – 2025 – 2032
The Global Needle-Free Injection System Market size is estimated to be valued at USD 3.8 billion in 2025 and is expected to reach USD 7.6 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 11.5% from 2025 to 2032.
Global Needle-Free Injection System Market Overview
Needle-free injection systems are innovative devices that deliver liquid medications or vaccines through high-pressure streams, bypassing traditional needles. These products are engineered to ensure precise dosage, reduce pain and fear associated with needles, and minimize the risk of needle-stick injuries. They are available in reusable and disposable designs, suitable for intradermal, subcutaneous, or intramuscular administration, and often include automated or spring-loaded mechanisms for consistency
Key Takeaways
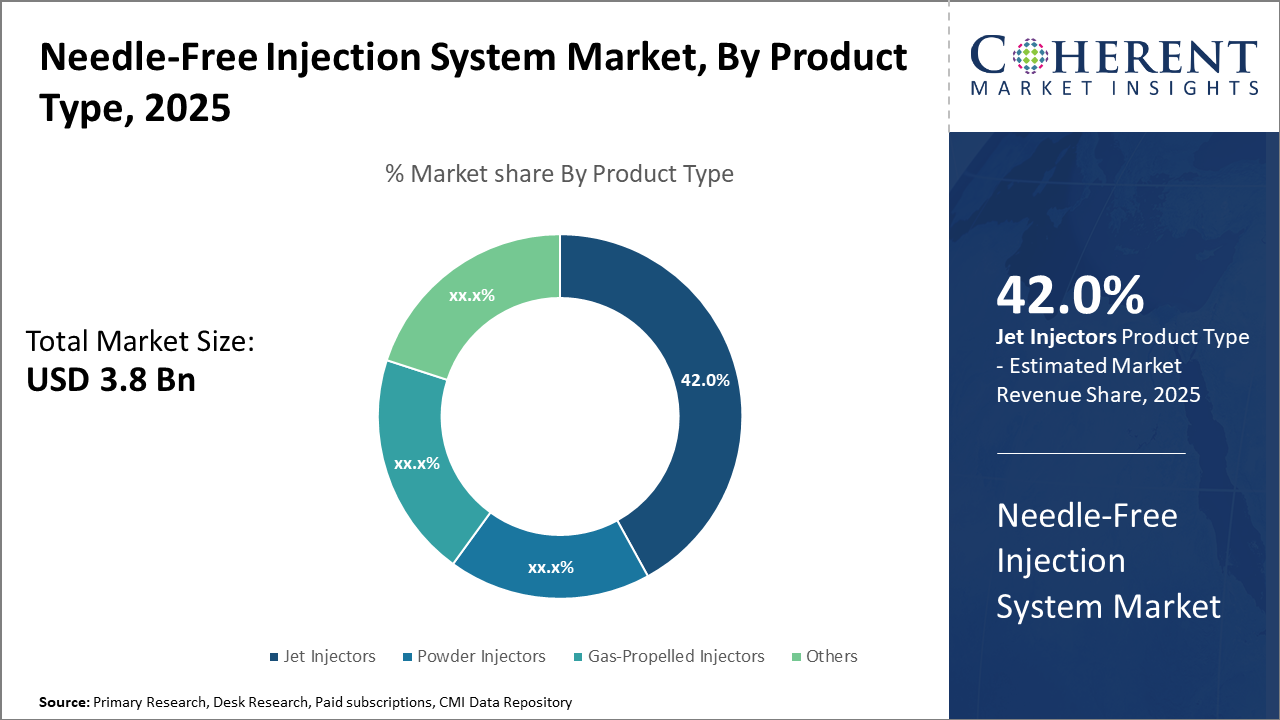
The Needle-Free Injection System market segment, dominated by jet injectors, continues to lead with approximately 42% share, driven by versatility and widespread application across vaccination and chronic therapies.
The vaccination application segment shows the fastest growth, fueled by expansive immunization programs and technological innovations improving delivery efficiency.
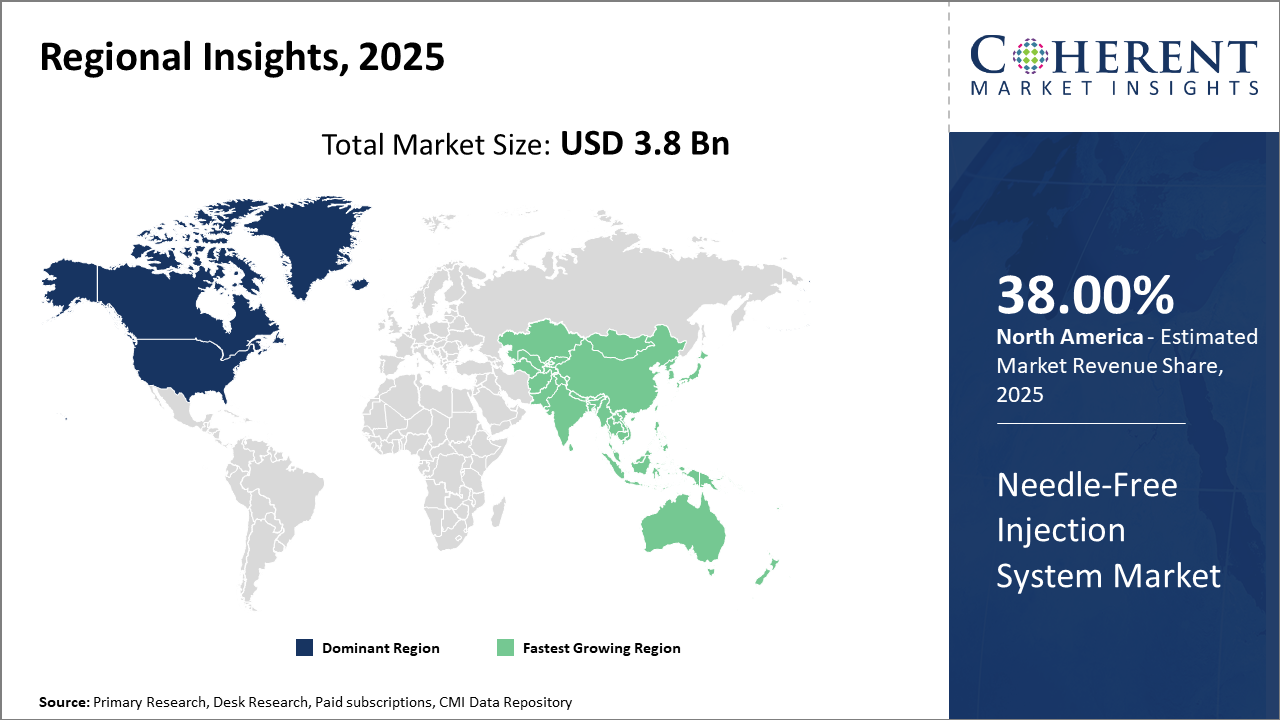
Regionally, North America holds the largest industry share, backed by advanced healthcare infrastructure and robust R&D investments from prominent market companies.
Asia Pacific is the fastest-growing region with a CAGR surpassing 14%, supported by government initiatives, a growing diabetic population, and expanding pharmaceutical manufacturing hubs in countries like India and China.
Higher adoption of needle-free injection systems in hospital settings marks a cornerstone for business growth, with increased patient safety protocols stimulating market revenue gains in key developed economies.
Emerging trends such as digital integration and hybrid delivery systems represent significant market growth strategies to watch, offering differentiation amidst intense market competition and evolving customer needs.
Needle-Free Injection System Market Segmentation Analysis
To learn more about this report, Download Free Sample
Needle-Free Injection System Market Insights, By Product Type
Jet Injectors dominate the market share at 42% due to their versatility across multiple therapy areas, excellent dose accuracy, and reusability, making them indispensable in vaccination drives and insulin delivery programs. Powder Injectors represent a fast-growing segment supported by innovation in dry powder vaccine delivery, increasing adoption in regions with cold-chain challenges. Gas-Propelled Injectors are commonly used in specialized applications such as hormone therapy, offering unique delivery benefits with a rapid onset.
Needle-Free Injection System Market Insights, By Application
Vaccination dominates the market share with over 38%, propelled by widespread immunization programs globally and the efficacy of needle-free injectors in mass immunizations. Insulin Delivery is the fastest-growing subsegment owing to the rising diabetic population and the shift towards minimally invasive injection solutions that reduce patient discomfort and improve compliance. Hormone Therapy includes applications in gender-affirming treatments and menopausal therapies, where injection comfort is a critical factor. Pain Management applications focus on delivering local anesthetics or analgesics without needles, expanding use in outpatient settings.
Needle-Free Injection System Market Insights, By End-User
Hospitals & Clinics hold the dominant market share of about 45%, attributable to institutional adoption driven by safety protocols, reducing needle-stick injuries, and improving injection accuracy in critical care settings. Home Healthcare is the fastest-growing subsegment, propelled by the rise of self-administration treatments and digital integration, facilitating remote patient monitoring. Research Laboratories utilize needle-free systems for experimental drug delivery and biologics testing, contributing to innovation-driven growth.
Needle-Free Injection System Market Trends
The Needle-Free Injection System market is undergoing a significant transformation catalyzed by three key trends.
Electronic jet injectors are gaining traction due to their higher dosing accuracy and user-friendliness compared to traditional pneumatic designs. For example, in 2025, electronic injector sales surged by 25% globally, driven by demand for precise medication delivery in chronic therapies.
The convergence of needle-free devices with digital and IoT technologies is creating new opportunities for patient adherence monitoring and personalized treatment plans. Pilot programs in North America demonstrated a 40% improvement in treatment outcomes using connected injectors.
Furthermore, hybrid drug delivery platforms capable of handling diverse biologics and vaccine formulations reflect another emerging shift, addressing the growing complexity of modern therapies and enabling expanded market scope.
Needle-Free Injection System Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Needle-Free Injection System Market Analysis and Trends
In North America, the dominance in the Needle-Free Injection System market is underscored by robust healthcare infrastructure, sizable government funding for medical device innovation, and widespread adoption in clinical practice. North America holds the largest market share globally, accounting for approximately 38% due to active participation from leading market companies like PharmaJet and Becton Dickinson. The region benefits from stringent safety regulations pushing needle-free adoption and well-established distribution networks.
Asia Pacific Needle-Free Injection System Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth, with a CAGR surpassing 14% propelled by expanding pharmaceutical manufacturing ecosystems, government initiatives to improve public health, and increasing prevalence of chronic diseases. Countries like India and China are pivotal, where significant investments and policy support catalyze expansion. PharmaJet’s partnerships with local firms and clinical acceptance in this region exemplify drivers contributing to rapid growth.
Needle-Free Injection System Market Outlook for Key Countries
USA Needle-Free Injection System Market Analysis and Trends
The USA stands as a critical hub dominating the Needle-Free Injection System market due to advancements in healthcare technology and strong regulatory support. The country recorded a 28% increase in hospital adoption rates in 2024, supported by initiatives aimed at minimizing needle-stick injuries and improving patient comfort. Major players such as Becton Dickinson and Enable Injections have invested heavily in R&D centers within the country to innovate next-generation injectors integrated with digital health technologies. These efforts have solidified the USA’s leadership position in market revenue and technological development.
India Needle-Free Injection System Market Analysis and Trends
India’s market is growing rapidly, driven by government public health campaigns and increasing biopharmaceutical activities. Needle-free injection systems are increasingly utilized in large-scale vaccination programs combating diseases like hepatitis and influenza, contributing to rising market share. Collaborations between international market players and domestic manufacturers have accelerated production capabilities and favorable pricing, expanding access to remote populations. This ecosystem, coupled with expanding home healthcare adoption, forecasts substantial needle-free injection system market growth for India in coming years.
Analyst Opinion
The rising adoption of needle-free injection systems in vaccine administration has been pivotal. Recent immunization programs have reported up to 30% reduction in vaccine wastage due to precise dosing capabilities of these systems in 2024, which strongly contributes to market size growth by reducing operation costs. Additionally, heightened regulatory endorsements for safety-compliant devices have streamlined market access.
The surge in home healthcare and self-administration therapies is significantly expanding demand. Pharmaceutical companies reported a 22% increase in production of self-injection devices equipped with needle-free technology in 2025, which is a critical demand-side indicator fueling market revenue expansion. The convenience and safety of needle-free solutions bolster their market share in chronic disease treatment.
Cost dynamics and pricing strategies are reshaping supply-side factors. Manufacturers have optimized production lines to cut costs by 15% in 2024, enabling more competitive pricing and broader adoption across low-to middle-income countries. This pricing trend has supported wider global penetration, enhancing the global market forecast.
Healthcare providers' growing preference for needle-free solutions due to minimized infection risk and better patient adherence is a key micro-indicator. Hospital procurement data from 2024 reveal a 28% spike in hospital-based needle-free injector purchases, attributing to safer injection practices and positively impacting market revenue and growth strategies.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 3.8 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 11.5% | 2032 Value Projection: | USD 7.6 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | PharmaJet, Antares Pharma, Bioject Medical Technologies, Enable Injections, Zogenix Inc., Becton Dickinson, West Pharmaceutical Services, Crossject, Crossject, Nemera, 3M Company, Gerresheimer AG, Aradigm Corporation, and Medimetrics | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Needle-Free Injection System Market Growth Factors
The needle-free injection system market growth is primarily fueled by the need to reduce needle-stick injuries and improve safety profiles within healthcare facilities worldwide. The World Health Organization reported a sharp increase in awareness campaigns in 2024, leading to policy shifts favoring needle-free technologies. Rapid advancements in drug delivery mechanisms that allow for better bioavailability and patient comfort also serve as critical drivers, with numerous biotech companies exploring needle-free platforms for biologics since 2023. Increasing chronic disease prevalence, such as diabetes, has further propelled demand, particularly for insulin delivery systems, as needle-free options dramatically reduce patient anxiety and improve adherence. Additionally, expansion of immunization programs across low- and middle-income countries has created new opportunities, with key vaccination drives utilizing needle-free systems to overcome logistical challenges in remote areas.
Needle-Free Injection System Market Development
In August 2025, NuGen Medical Devices unveiled its new internal cartridge system, designed to enhance the performance and usability of its needle-free injection platform. The advanced cartridge integrates a sealed, prefilled mechanism that simplifies medication loading, ensures precise dosage delivery, and minimizes contamination risks during administration. Compatible with multiple drug formulations, the system supports applications across insulin delivery, pain management, and vaccine administration.
In November 2024, Sol-Millennium Medical Group introduced the Inset™ for insulin delivery, an innovative needle-free injector system designed specifically for diabetes management. The Inset device uses controlled pressure technology to deliver insulin subcutaneously without the need for traditional needles, ensuring painless, precise, and consistent dosing. Engineered with ergonomic design and single-use safety mechanisms, the Inset offers diabetic patients a discreet, hygienic, and sustainable alternative to conventional insulin pens and syringes.
Key Players
Leading Companies of the Market include PharmaJet, Antares Pharma, Bioject Medical Technologies, Enable Injections, Zogenix Inc., Becton Dickinson, West Pharmaceutical Services, Crossject, Crossject, Nemera, 3M Company, Gerresheimer AG, Aradigm Corporation, and Medimetrics, among others.
Some major market players are adopting competitive strategies centered on innovation and strategic partnerships. For instance, PharmaJet expanded its production capacity in 2024 by 40% after entering agreements with vaccine manufacturers for large-scale deployment. Similarly, Bioject Medical Technologies’ launch of a next-gen needle-free injector with integrated digital monitoring significantly improved user adherence in clinical trials, driving its market share uptick by nearly 10% in 2025. Strategic acquisitions, such as Antares Pharma acquiring niche device startups, enabled faster access to new technologies and diversified product portfolios.
Needle-Free Injection System Market Future Outlook
The market is expected to grow rapidly as healthcare providers increasingly adopt needle-free systems for vaccines, biologics, and insulin delivery. Enhanced designs focusing on dose accuracy, portability, and usability will support adoption in clinical and home care settings. Government vaccination initiatives, pediatric healthcare programs, and rising patient preference for pain-free delivery methods will further drive market expansion. Integration with digital tracking and IoT-enabled devices may improve compliance and monitoring, strengthening market prospects globally.
Needle-Free Injection System Market Historical Analysis
Needle-free injection systems emerged as a safer, more convenient alternative to traditional needle-based delivery methods. Initially, adoption was limited to specialized healthcare settings and certain vaccine campaigns. Over time, technological improvements in jet injectors and spring-loaded delivery mechanisms increased reliability, precision, and patient compliance. Needle-free devices addressed critical issues such as needle-stick injuries, cross-contamination, and patient anxiety, which contributed to gradual market acceptance. Regulatory approvals and growing emphasis on vaccination programs accelerated adoption in both developed and developing regions.
Sources
Primary Research interviews:
Biomedical Engineers
Pharmacologists
Immunologists
Device Manufacturers
Healthcare Practitioners
Databases:
FDA Medical Device Database
PubMed
Magazines:
Drug Delivery Business News
Medical Device Network
PharmaVoice
MedTech Innovation News
Journals:
Journal of Drug Delivery Science and Technology
International Journal of Pharmaceutics
Expert Opinion on Drug Delivery
Advanced Drug Delivery Reviews
Newspapers:
The New York Times (Health)
The Guardian (Science)
The Economic Times (Healthcare)
The Washington Post (Health)
Associations:
Drug Delivery Alliance
World Health Organization (WHO)
International Society for Pharmaceutical Engineering (ISPE)
U.S. FDA
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients