Needle Free Blood Drawing Devices Market Size and Forecast – 2026 – 2033
The Global Needle Free Blood Drawing Devices Market size is estimated to be valued at USD 0.75 billion in 2026 and is expected to reach USD 1.35 billion by 2033, exhibiting a compound annual growth rate (CAGR) of 9.3% from 2026 to 2033.
Global Needle Free Blood Drawing Devices Market Overview
The Needle Free Blood Drawing Devices market offers innovative solutions designed to collect blood samples without traditional needles, improving patient comfort and safety. Products include jet injectors, vacuum-based collection systems, and micro-needle technologies that enable rapid, minimally invasive blood sampling. Devices are often compatible with standard laboratory testing protocols and integrate features such as single-use cartridges, automated collection, and ergonomic designs for ease of use by healthcare professionals. Key applications span hospitals, diagnostic laboratories, and outpatient clinics, with emphasis on reducing needle-stick injuries, minimizing patient anxiety, and enhancing workflow efficiency in clinical and point-of-care settings.
Key Takeaways
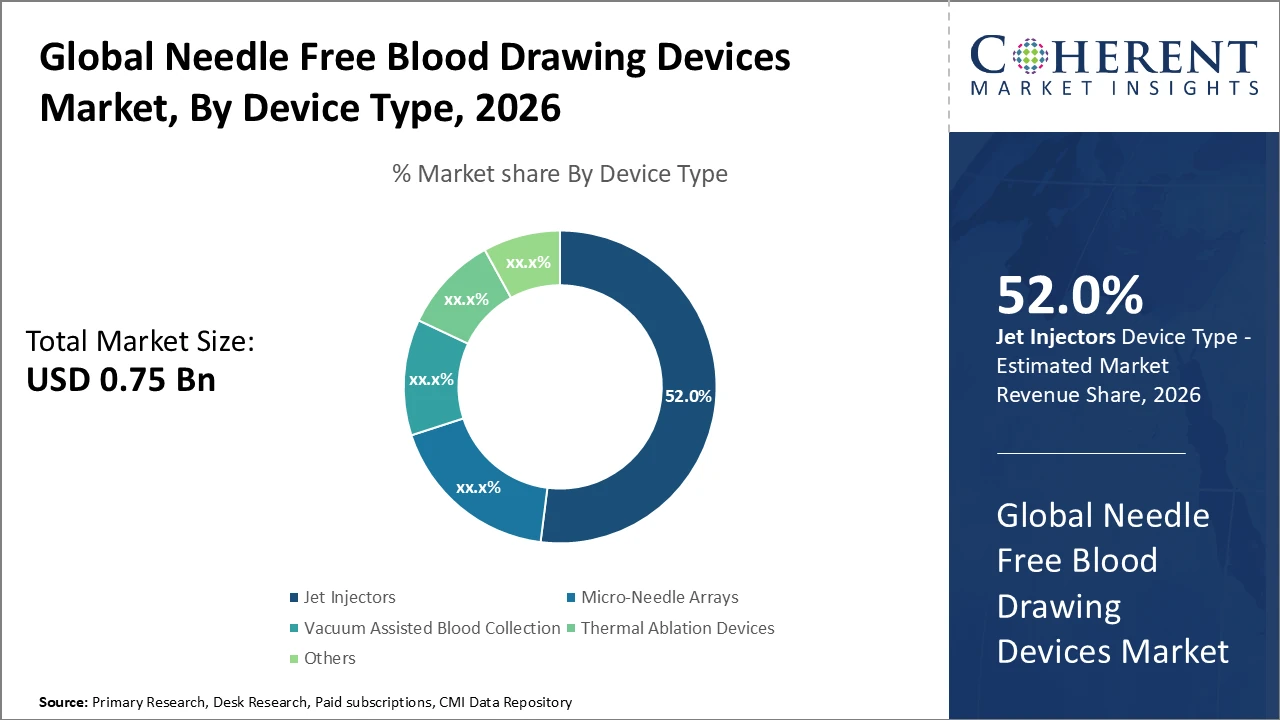
The Jet Injectors segment dominates the Needle Free Blood Drawing Devices market, holding 52% of the industry share in 2026, driven by high accuracy and enhanced user comfort.
Hospitals are the largest end-user segment, fueled by strong demand for sterile, efficient, and safe blood collection processes.
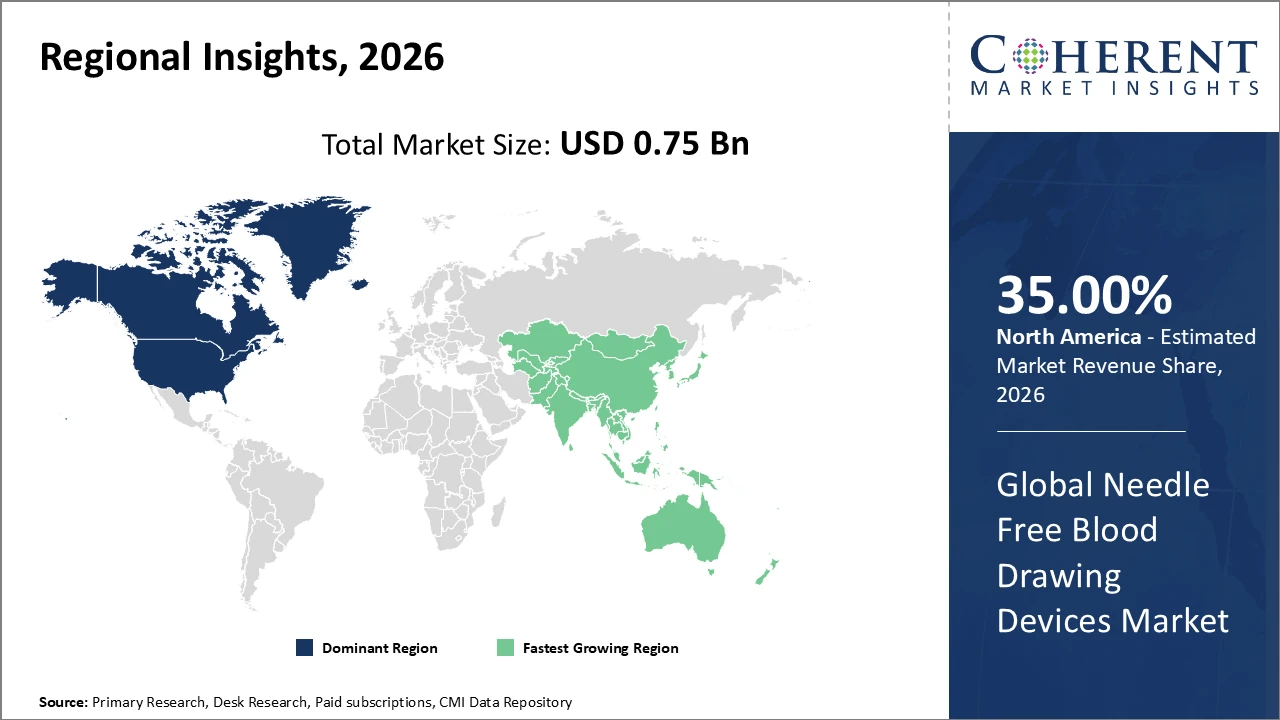
North America leads the regional market with over 35% share, supported by advanced healthcare infrastructure, high technology adoption, and favorable regulatory frameworks.
Asia Pacific is the fastest-growing region, recording a CAGR of 11.2% from 2024 to 2026, driven by expanding healthcare services, increasing patient awareness, and growing adoption of minimally invasive technologies.
Needle Free Blood Drawing Devices Market Segmentation Analysis
To learn more about this report, Download Free Sample
Needle Free Blood Drawing Devices Market Insights, By Device Type
Jet Injectors dominate the Needle Free Blood Drawing Devices market, accounting for 52% of the share, as they provide precise, painless blood collection without skin penetration, making them highly favored in hospitals and clinics. The fastest-growing subsegment is Micro-Needle Arrays, driven by their minimally invasive approach and compatibility with wearable diagnostic technologies. Vacuum Assisted Blood Collection systems maintain steady demand for routine clinical applications, while Thermal Ablation Devices represent a niche but emerging segment due to their combined therapeutic and diagnostic capabilities. Other devices serve specialized clinical functions. Overall, the focus on patient comfort, safety, and infection control continues to drive growth across all subsegments.
Needle Free Blood Drawing Devices Market Insights, By Application
Diabetic testing dominates the Needle Free Blood Drawing Devices market, accounting for over 45% of the share, driven by the growing global diabetic population that requires frequent blood sampling without needle-related discomfort. Oncology monitoring is the fastest-growing application segment, fueled by the increasing number of cancer survivors needing regular, painless testing. Pediatrics holds a significant share due to the demand for gentle blood collection methods in children. Emergency medicine employs these devices for rapid, infection-free sampling in critical care settings, while the Others segment includes applications for infectious disease testing and routine health check-ups.
Needle Free Blood Drawing Devices Market Insights, By End-User
Hospitals remain the dominant end-user segment in the Needle Free Blood Drawing Devices market, holding over 60% share in 2026, due to their extensive use of advanced devices for both routine and emergency blood collection. The fastest-growing subsegment is Home Healthcare, driven by rising chronic disease prevalence and patient preference for self-administered monitoring, especially in diabetes care. Diagnostic laboratories maintain steady demand by focusing on high-throughput testing, while clinics hold a moderate share, primarily serving outpatient services. The Others category includes specialized care centers adopting needle-free technologies in situations where conventional methods are less effective or pose challenges.
Needle Free Blood Drawing Devices Market Trends
Recent years have seen a strong shift toward integration with digital health platforms, with needle free blood drawing devices connecting to IoT-enabled diagnostics for real-time patient data transmission and monitoring.
In 2026, several U.S. hospitals implemented smart devices with wireless data upload capabilities, improving workflow efficiency and enhancing patient care.
Device customization for specific patient demographics is emerging as a key trend, exemplified by pediatric-friendly designs launched in 2025, helping manufacturers capture niche market segments.
Growing environmental awareness has increased focus on sustainability, driving innovations such as biodegradable components introduced in 2024.
These trends are reshaping market dynamics and influencing global business growth models in the needle free blood drawing devices sector.
Needle Free Blood Drawing Devices Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Needle Free Blood Drawing Devices Market Analysis and Trends
In North America, the Needle Free Blood Drawing Devices market is dominated by a well-established healthcare ecosystem, supportive regulatory frameworks, and strong R&D investments. The region held over 35% of the global market share in 2026. Leading companies such as BD and Retractable Technologies are headquartered in North America, driving innovation and contributing significantly to market revenue. Government initiatives promoting safer, needle-free medical devices have accelerated adoption, establishing the region as a mature and highly lucrative market for these technologies.
Asia Pacific Needle Free Blood Drawing Devices Market Analysis and Trends
Asia Pacific is the fastest-growing market for Needle Free Blood Drawing Devices, recording a CAGR of 11.2%, driven by expanding healthcare infrastructure, rising disposable incomes, and growing patient awareness. Rapid urbanization and healthcare digitization in countries like India and China are fueling demand for these devices. Supportive government policies, including incentives for medical device manufacturing and relaxed import regulations, further accelerate market growth. Collaborations between local players and global companies have strengthened distribution networks and product availability, contributing significantly to the region’s expanding market presence.
Needle Free Blood Drawing Devices Market Outlook for Key Countries
USA Needle Free Blood Drawing Devices Market Analysis and Trends
The USA market continues to be a key driver in the Needle Free Blood Drawing Devices sector, fueled by healthcare providers’ efforts to reduce needlestick injuries. In 2025, approximately USD 250 million was invested in developing and adopting advanced jet injector technologies. Companies like BD have driven market growth through innovative, FDA-compliant product launches, expanding their presence across hospitals and outpatient facilities. Additionally, the integration of digital health platforms enabling connected care has reinforced the USA’s leading position, sustaining strong market revenue and share contribution on a global scale.
Germany Needle Free Blood Drawing Devices Market Analysis and Trends
Germany’s Needle Free Blood Drawing Devices market is driven by advanced healthcare infrastructure, strong regulatory oversight, and a focus on patient safety and infection control. Hospitals and diagnostic centers are the primary adopters, integrating jet injectors and micro-needle arrays to enhance efficiency and minimize needle-related risks. Recent trends include digital integration with hospital IT systems for real-time data monitoring and pediatric-focused device designs. Sustainability is also gaining traction, with biodegradable and energy-efficient components entering the market. Government initiatives promoting medical device innovation and stringent hygiene standards further support adoption, positioning Germany as a key contributor to the European market’s growth and technological advancement.
Analyst Opinion
Increasing production capacity and innovation: Manufacturers boosted production capabilities by over 20% in 2025 to meet rising demand for needle-free blood devices. For example, a leading European manufacturer expanded its facility, achieving a 30% increase in quarterly output in early 2026, directly contributing to market share growth.
Rising usage in chronic disease management: Adoption of needle-free devices for diabetic and oncology patients has grown significantly due to safety and ease of use. Data from 2024 indicates a 15% increase in device utilization within outpatient clinics, reinforcing demand-side market drivers.
Pricing trends and affordability enhancements: Average device costs decreased by 5% in 2025 through economies of scale and material innovations, making advanced devices more accessible and supporting increased penetration in cost-sensitive emerging markets.
Expanding import and export dynamics: Cross-border shipments increased by 12% in 2026, with exports from North America and Asia rising to meet demand in Latin America and the Middle East, boosting global revenue and supporting market expansion.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 0.75 million |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 9.3% | 2033 Value Projection: | USD 1.35 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | BD, Terumo Corporation, Unilife Corporation, Nipro Corporation, Owen Mumford plc, Mylan N.V., Smiths Medical, Vascular Solutions, Inc., HemoCue AB, Essentia Health | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Needle Free Blood Drawing Devices Market Growth Factors
The growing prevalence of chronic diseases worldwide has driven strong demand for pain-free blood sampling, boosting the Needle Free Blood Drawing Devices market. Health reports from 2025 indicate that diabetes monitoring using these devices rose by nearly 22%. Technological innovations such as micro-needling and jet injector systems enhance safety and user convenience, encouraging adoption in outpatient clinics and home healthcare. Regulatory incentives promoting safer medical devices in North America and Europe have accelerated adoption, contributing to approximately 15% revenue growth in these regions. Expanding healthcare infrastructure and rising patient awareness in Asia Pacific have further fueled market expansion, supporting a 12% CAGR in device sales from 2024 to 2026.
Needle Free Blood Drawing Devices Market Development
In November 2023, Becton, Dickinson and Company, a global medical technology leader, launched a new needle-free blood collection technology designed for use with integrated catheters, advancing its vision of a “One-Stick Hospital Stay.”
Key Players
Leading Companies of the Market
BD
Terumo Corporation
Unilife Corporation
Nipro Corporation
Owen Mumford plc
Mylan N.V.
Smiths Medical
Vascular Solutions, Inc.
HemoCue AB
Essentia Health
Competitive strategies in the Needle Free Blood Drawing Devices market include BD’s expansion through technology licensing agreements in the Asia Pacific region, which led to a 14% increase in market revenue in 2025. Terumo Corporation focused on R&D for micro-needle jet injectors, gaining a 10% market share in 2026 by providing devices with improved patient comfort and usability. Similarly, Retractable Technologies strengthened its presence in Latin America through strategic partnerships, reducing product delivery lead times and boosting market penetration by 18%, demonstrating how collaborations and innovation are key drivers in the competitive landscape.
Needle Free Blood Drawing Devices Market Future Outlook
The Needle Free Blood Drawing Devices market is poised for robust growth, driven by rising chronic disease prevalence, increasing demand for pain-free sampling, and growing adoption in home healthcare and outpatient settings. Future trends include advanced micro-needle arrays, AI-enabled smart devices, and integration with digital health platforms for real-time patient monitoring. Sustainability initiatives, such as biodegradable components, will further shape product development. Emerging markets in Asia Pacific and Latin America are expected to witness rapid expansion due to improving healthcare infrastructure and rising patient awareness. Overall, innovation, safety, and convenience will continue to define market growth and adoption globally.
Needle Free Blood Drawing Devices Market Historical Analysis
The Needle Free Blood Drawing Devices market has experienced steady growth over the past decade, driven by the need for safer, painless, and infection-free blood collection methods. Early adoption was led by hospitals and diagnostic laboratories in North America and Europe, focusing on jet injectors and vacuum-assisted systems. Technological advancements gradually introduced micro-needle arrays and pediatric-friendly designs, improving usability and patient compliance. Market expansion was supported by regulatory emphasis on needlestick injury prevention and increasing chronic disease prevalence. Historical growth patterns set the stage for digital integration, home healthcare adoption, and emerging market penetration, establishing a strong foundation for current and future market trends.
Sources
Primary Research Interviews:
Hospital IT Mnagers
Nurses and Clinicians
Biomedical Engineers
Medical device Manufacturers
Databases:
WHO Health Statistics
OECD Health Data
Global Health Observatory
Magazines:
Medical Design & Outsourcing
Healthcare IT News
Healthcare Innovation
HealthTech Magazine
Medical Device Network
Journals:
Journal of Medical Systems
Health Journal Informatics
Applied Clinical Informatics
International Journal of Medical Informatics
Newspapers:
The New York Times (Health)
The Guardian (Health)
Financial Times (Healthcare)
The Hindu (Health)
Reuters Health
Associations:
Healthcare Information and Management Systems Society (HIMSS)
American Hospital Association (AHA)
Medical Device Manufacturers Association (MDMA)
International Electrotechnical Commission (IEC – Medical Devices)
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients