Nasal Atomizer Devices Market Size and Forecast – 2026 – 2033
The Global Nasal Atomizer Devices Market size is estimated to be valued at USD 1.2 billion in 2026 and is expected to reach USD 2.3 billion by 2033, exhibiting a compound annual growth rate (CAGR) of 9.2% from 2026 to 2033.
Global Nasal Atomizer Devices Market Overview
Nasal atomizer devices are medical devices designed to deliver medications into the nasal cavity in the form of a fine mist. These devices enable rapid absorption through the nasal mucosa, bypassing the gastrointestinal tract and first-pass metabolism. Products are used for administering vaccines, pain medications, emergency drugs such as naloxone, and treatments for allergies or migraines. They are available in single-use or reusable formats and are designed for accuracy, ease of use, and patient comfort.
Key Takeaways
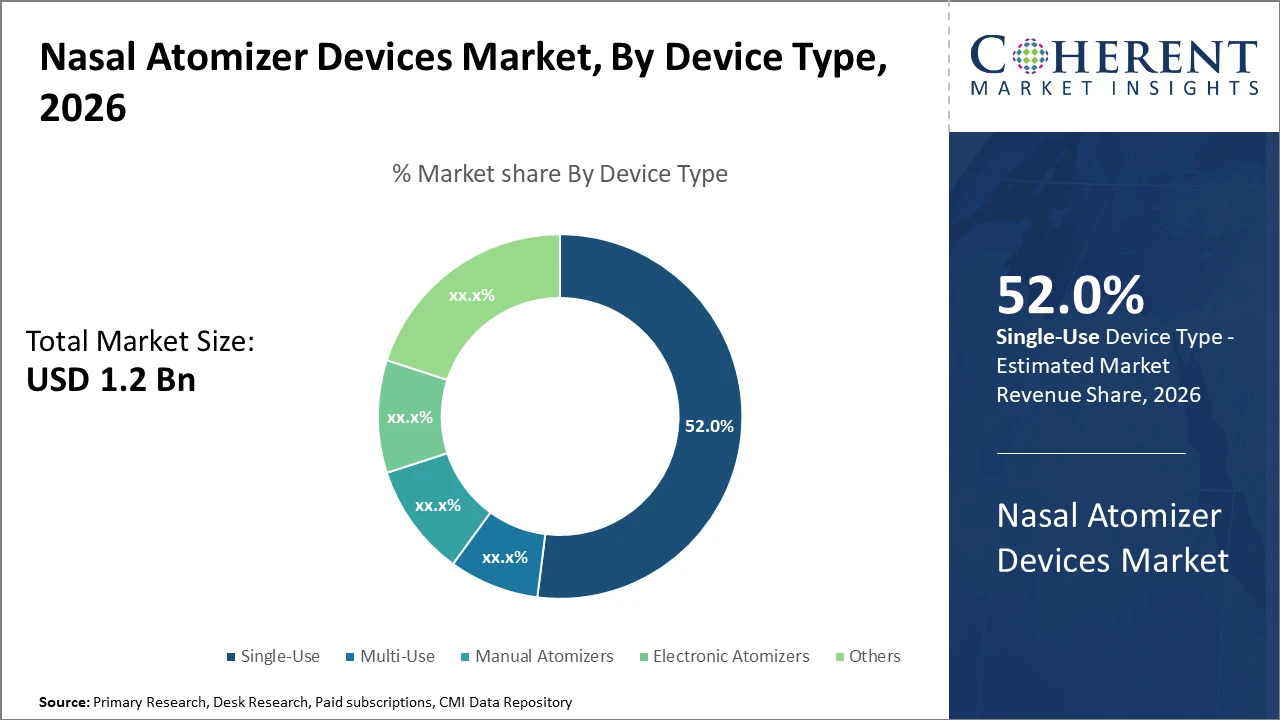
The Single-Use device segment commands over half of the market share due to its convenience and infection control advantages noted across healthcare settings by 2026.
Pharmaceutical drug delivery remains the leading application segment, comprising approximately 60% of industry share, driven by rising chronic disease management needs.
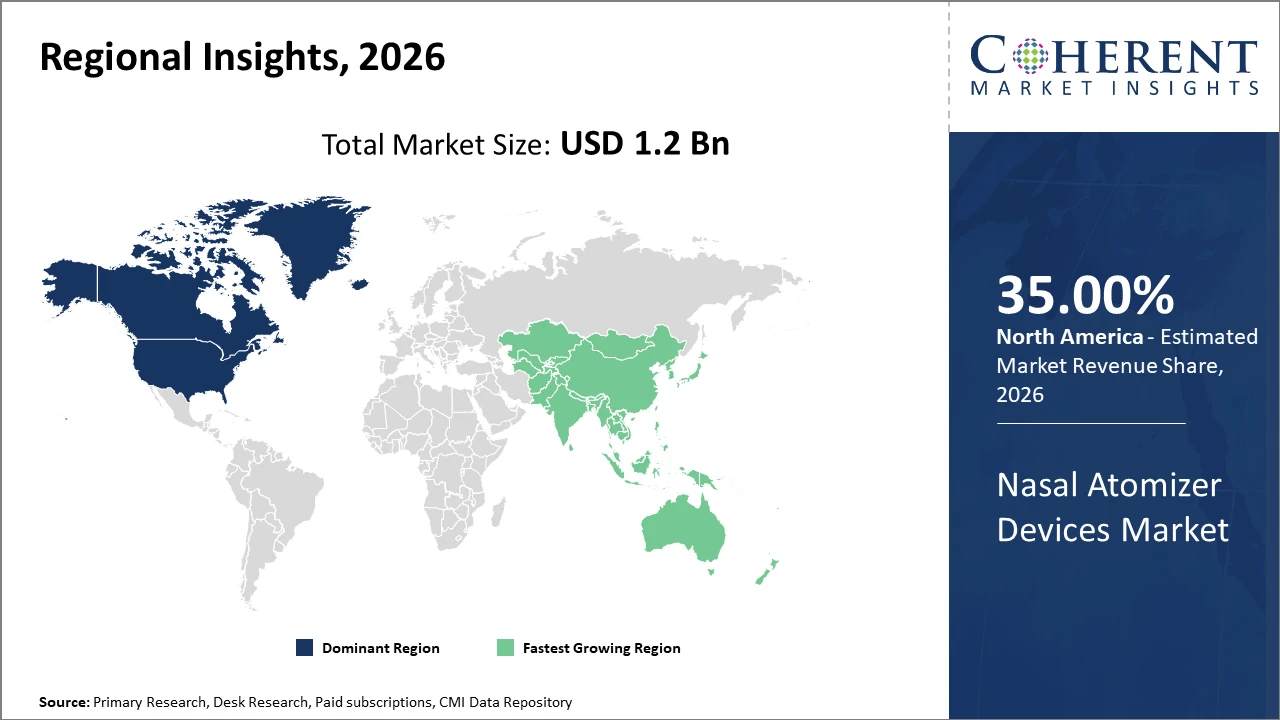
North America dominates with around 35% market share, buoyed by advanced healthcare infrastructure and regulatory facilitation.
Asia Pacific exhibits the fastest CAGR, exceeding 11%, propelled by expanding healthcare access, growing pharmaceutical manufacturing bases, and increasing awareness of nasal drug delivery benefits.
Nasal Atomizer Devices Market Segmentation Analysis
To learn more about this report, Download Free Sample
Nasal Atomizer Devices Market Insights, By Device Type
Single-use devices dominate due to their sterility, convenience, and regulatory preference, especially within hospital environments where infection control is critical. This subsegment benefits from streamlined supply chains and high patient turnover. The fastest-growing subsegment is Electronic Atomizers, which incorporate advanced features such as dose control and IoT connectivity, enhancing user adherence and real-time monitoring capabilities. Manual Atomizers retain relevance due to cost-effectiveness and simplicity, finding significant utilization in home care and veterinary sectors. Multi-use devices occupy niche applications requiring repeat dosing but face constraints owing to sterilization challenges. Others include specialized atomizer types designed for cosmetic or research use, each offering tailored functionalities.
Nasal Atomizer Devices Market Insights, By Application
Pharmaceutical Drug Delivery holds the largest share, roughly 60%, driven by chronic respiratory treatments and pain management, where rapid onset is crucial. Innovations in drug formulation compatible with nasal atomization have broadened therapeutic avenues. Nasal Vaccination displays the fastest growth due to expanded immunization programs especially visible in Asia Pacific and Europe post-pandemic, supported by favorable government initiatives. Cosmetic & Personal Care applications leverage nasal atomizers for aroma therapy and skin treatments, capturing niche consumer markets. Veterinary Use is expanding steadily, benefiting from animal health awareness and easier drug administration protocols.
Nasal Atomizer Devices Market Insights, By End-User
Hospitals & Clinics dominate market revenue, attributed to their high volume usage and priority on sterile, single-use devices aligned with patient safety standards. They also spearhead the adoption of innovative products integrating digital health features. Homecare Settings exhibit the fastest growth due to rising chronic disease prevalence and patient preference for home-based nasal atomization therapies. Veterinary Clinics are increasingly adopting advanced nasal atomizer devices as awareness of animal welfare and medication delivery improves. Research Institutes contribute through device testing and development of novel applications, while Others include cosmetic establishments and specialized treatment centers, providing a diversified market scope.
Nasal Atomizer Devices Market Trends
Recent industry trends highlight a growing emphasis on integrating nasal atomizer devices with IoT technologies to improve patient adherence and data analytics.
For instance, Teleflex’s 2026 release of a digitally connected atomizer enhanced treatment monitoring in respiratory therapies.
Another key trend is the surge in nasal vaccines, propelled by pandemic preparedness initiatives, driving rapid market acceptance and innovative product pipelines.
Sustainable device manufacturing is also gaining momentum, with companies adopting biodegradable components to address environmental concerns, evidenced by a 22% reduction in plastic usage in newly launched products by 2025.
Nasal Atomizer Devices Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Nasal Atomizer Devices Market Analysis and Trends
In North America, the dominance in the Nasal Atomizer Devices market is attributed to robust healthcare infrastructure, supportive regulatory frameworks, and high R&D investments. The U.S. leads with significant pharmaceutical manufacturing presence and advanced distribution networks. Market companies such as BD and AptarGroup have capitalized on these strengths, boosting market share to approximately 35%. Regulatory facilitation from the FDA for nasal vaccine approvals has further accelerated growth.
Asia Pacific Nasal Atomizer Devices Market Analysis and Trends
Meanwhile, Asia Pacific exhibits the fastest growth, with a CAGR exceeding 11%, driven by expanding healthcare access, increasing patient awareness, and government initiatives supporting the medical device sectors. Countries like China and India are emerging as manufacturing hubs, while rising chronic respiratory disease prevalence fuels demand. Regional players and multinational market companies have intensified collaborations, leading to broader market penetration.
Nasal Atomizer Devices Market Outlook for Key Countries
USA Nasal Atomizer Devices Market Analysis and Trends
The U.S. maintains a pivotal role given extensive pharmaceutical R&D, advanced technological adoption, and a high preference for non-invasive treatment solutions. Notably, telehealth expansion post-2024 has spurred demand for smart nasal atomizers compatible with remote patient monitoring, contributing to a steady increase in market revenue. Leading market companies focus heavily on FDA approvals and innovation-driven launches, reinforcing leadership status.
India Nasal Atomizer Devices Market Analysis and Trends
India's Nasal Atomizer Devices market is expanding rapidly due to growing healthcare infrastructure investment and increased consumer consciousness around respiratory health. Government schemes facilitating healthcare device manufacturing and import regulation relaxations in 2025 catalyzed business growth. Local companies are partnering with global players, enhancing production capacity and adapting cost-effective solutions suited for home care and veterinary applications, thereby accelerating regional market growth.
Analyst Opinion
The surge in pharmaceutical applications of nasal atomizer devices forms a critical demand-side indicator driving market size. Recent adoption in allergy and pain management treatments has increased market penetration by approximately 15% in 2025, reflecting user preference for rapid mucosal drug delivery.
Supply-side dynamics, particularly production capacity enhancements, have notably influenced market growth. For example, manufacturing expansions reported by leading healthcare manufacturers in 2024 led to a 12% increase in device availability worldwide, enabling broader product launches.
Pricing strategies within emerging economies have supported higher adoption rates; competitive pricing in India and Southeast Asia increased imports by 18% in 2026, facilitating market diversification across the Asia Pacific.
Uses across diverse industries like veterinary medicine and cosmetic applications also contribute to nano-scale market expansion. Notably, veterinary use accounted for a 7.5% rise in overall market share in 2025, signaling widening market segments beyond traditional healthcare.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: |
USD 1.2 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 9.2% | 2033 Value Projection: | USD 2.3 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | AptarGroup Inc., Teleflex Incorporated, Koninklijke Philips N.V., BD (Becton Dickinson and Company), Medspray AS, 3M Company, Nordson Corporation, Vectura Group Plc, Nipro Corporation, Catalent Inc. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Nasal Atomizer Devices Market Growth Factors
Increased prevalence of chronic respiratory diseases has been a pivotal driver, creating steady demand for effective nasal drug delivery solutions. For instance, WHO data from 2025 suggests chronic respiratory ailments affected over 10% of the global population, fueling the increase in nasal atomizer usage. Regulatory approvals expedited in 2026, particularly in North America and Europe, have expanded market scope by streamlining product availability. Growing patient preference for non-invasive, needle-free drug delivery methods continues to push adoption rates upward, supported by the convenience and enhanced bioavailability nasal atomization offers. Technological advancements, including improvements in atomizing nozzles and electronic device integration, have improved dosing precision and user experience, reported widely across major pharmaceutical companies in 2024 and 2025.
Nasal Atomizer Devices Market Development
In August 2024, Aptar Pharma launched its Unidose® nasal delivery system for Neffy (epinephrine), a needle-free treatment for anaphylaxis. The launch highlights a single-use, ready-to-administer nasal device designed to deliver a precise dose of epinephrine rapidly and reliably, addressing patient anxiety and usability challenges associated with injectable auto-injectors while improving accessibility and adherence in emergency allergy care.
In 2024, Alcove and Pulmodyne entered into a strategic collaboration to expand distribution and advance innovation in nasal atomization technologies through the EZ-Spray® system. This partnership focuses on broadening market reach while jointly researching next-generation atomization solutions that enhance drug dispersion and delivery efficiency, supporting improved therapeutic outcomes across respiratory and nasal drug administration applications.
Key Players
Leading Companies of the Market
AptarGroup Inc.
Teleflex Incorporated
Koninklijke Philips N.V.
BD (Becton Dickinson and Company)
Medspray AS
3M Company
Nordson Corporation
Vectura Group Plc
Nipro Corporation
Catalent Inc.
Several market players have undertaken strategic collaborations and mergers to solidify competitive advantage. For instance, Teleflex’s acquisition of specialized device manufacturers in 2025 enhanced its production portfolio, improving market share by 4.3% in North America. Meanwhile, AptarGroup’s investment in R&D focusing on biodegradable components resulted in a 2026 product launch that captured significant market interest, elevating its global footprint.
Nasal Atomizer Devices Market Future Outlook
Future growth in the nasal atomizer devices market is expected to be driven by expanding use cases beyond traditional therapies, including neurological treatments, biologics delivery, and emergency response medications. Advances in formulation science and device engineering are likely to improve bioavailability and patient compliance. As healthcare systems increasingly prioritize non-invasive and self-administered therapies, nasal atomizers are expected to gain wider acceptance in both clinical and home-care settings. Regulatory support for alternative delivery routes and ongoing innovation in device miniaturization will further strengthen market prospects.
Nasal Atomizer Devices Market Historical Analysis
The nasal atomizer devices market has its roots in early intranasal drug delivery systems developed to address limitations associated with oral and injectable administration routes. Initially, nasal sprays were primarily used for allergy and decongestant treatments, with relatively simple pump-based designs. Over time, clinical research highlighted the nasal cavity’s potential for rapid systemic absorption and direct nose-to-brain delivery, leading to expanded applications in pain management, emergency medicine, and vaccination. Technological improvements in spray pattern control, dose accuracy, and device ergonomics gradually transformed nasal atomizers into precision drug delivery tools suitable for both prescription and emergency use.
Sources
Primary Research Interviews:
ENT specialists
emergency physicians
device manufacturers
Pharmacists
respiratory therapists
Databases:
FDA Device Approvals
WHO Respiratory Health Data
OECD Health Data
Magazines:
Medical Device Network
ENT Today
Pharmaceutical Technology
Healthcare Weekly
MedTech Insight
Journals:
International Forum of Allergy & Rhinology
Respiratory Medicine
Drug Delivery and Translational Research
Journal of Aerosol Medicine
Clinical Otolaryngology
Newspapers:
Reuters Health
Financial Times (Healthcare)
The Guardian (Health)
The New York Times (Medicine)
Bloomberg Medical
Associations:
American Academy of Otolaryngology
European Respiratory Society
WHO
AdvaMed
International Society for Aerosols in Medicine
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients