Nasal drug delivery system is an alternative to invasive administrations and provides direct access to systemic circulation. Intranasal drug delivery is common and most preferred route of administration in treatment of allergic and non-allergic rhinitis, pain, acute treatment of migraine, and nasal congestion associated with sinusitis, common cold or rhinitis. Nasal drug delivery systems are mostly used in case wherein rapid onset of action is required, or to reach the site where other routes of administration display ineffective results. Nasal drug delivery is also preferred route for needle-free vaccination and systemic drug delivery. Liquid nasal preparations can be administered through inhalers, droppers, spray pumps, nebulizers or atomizers.
Global nasal drug delivery systems market is estimated to be valued at US$ 60.3 million in 2022 and is expected to exhibit a CAGR of 6.3% during the forecast period (2022-2030).
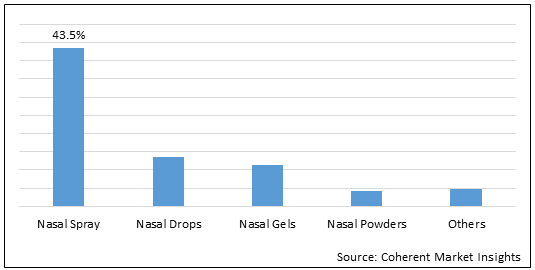
Figure 1. Global Nasal Drug Delivery Systems Market Share (%), by Dosage Form, 2022
To learn more about this report, Download Free Sample
Increasing product approvals by regulatory authorities is expected to drive growth of the global nasal drug delivery systems market.
Increasing product approvals by regulatory authorities is expected to drive growth of the global nasal drug delivery systems market. For instance, on March 18, 2019, Aptar Pharma, a drug delivery systems provider, had announced that the company’s product Bidose nasal spray device had received approval from the U.S. Food and Drug administration (FDA) for a breakthrough therapy in the field of depression.
Nasal Drug Delivery Systems Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 60.3 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 6.3% | 2030 Value Projection: | US$ 98.4 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Merck & Co., Inc., GlaxoSmithKline plc, AstraZeneca plc, Johnson & Johnson, Pfizer Inc., Baxter International, Inc., Valeant Pharmaceuticals International, Inc., Becton, Dickinson and Company, and Novartis AG |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
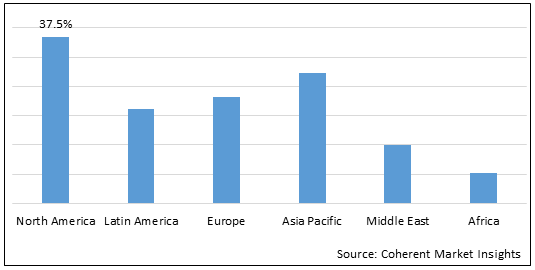
Figure 2.Global Nasal Drug Delivery Systems Market Share (%), by Region, 2022
To learn more about this report, Download Free Sample
Increasing product launches by market players is expected to drive the global nasal drug delivery systems market growth.
Increasing product launches by market players is expected to drive the market growth over the forecast period. For instance, on December 22, 2021, Teva Pharmaceuticals, a U.S. affiliate of Teva Pharmaceutical Industries Ltd.), announced the launch of generic version of Narcan1 (naloxone hydrochloride nasal spray). Moreover, naloxone hydrochloride nasal spray is a prescription medicine used for the treatment of an opioid emergency such as an overdose or a possible opioid overdose with signs of breathing problems or inability to respond.
Global Nasal Drug Delivery Systems Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe and the World Health Organization declared it a public health emergency on January 30, 2020.
The COVID-19 pandemic had a positive impact on the global nasal drug delivery systems market, owing to increased product launches by market players for protecting patients from coronavirus infection. For instance, on February 09, 2022, Glenmark Pharmaceuticals Limited, a global pharmaceutical company and Canada based pharmaceutical company SaNOtize Research & Development Corp., announced launch of its Nitric Oxide Nasal Spray under the brand name FabiSpray in India for the treatment of adult patients with COVID-19 who have high risk of progression of the disease.
Global Nasal Drug Delivery Systems Market: Key Developments
On April 28, 2022, Birmingham Biotech, a company that offers a wide variety of in vitro diagnostic solutions, medical devices and equipment, announced the Singapore launch of its latest technology the BHM Anti-Viral Nasal Spray, an easy-to-use nasal device with a unique patented formulation, Norizite, designed to help prevent infection from the inhalation of airborne viruses within the nasal passages.
Global Nasal Drug Delivery Systems Market: Restraint
Limitations associated with nasal drug delivery system such as histological toxicity or lesser area of absorption as compared to orally administered drug hinders the nasal drug delivery systems market growth.
Key Players
Major players operating in the global nasal drug delivery systems market include Merck & Co., Inc., GlaxoSmithKline plc, AstraZeneca plc, Johnson & Johnson, Pfizer Inc., Baxter International, Inc., Valeant Pharmaceuticals International, Inc., Becton, Dickinson and Company, and Novartis AG.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients