Global lupus therapeutic market is estimated to be valued at USD 3.55 Bn in 2025 and is expected to reach USD 6.53 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 9.1% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
The global lupus therapeutic market is expected to witness a positive growth trend over the forecast period. There is an increasing prevalence of lupus worldwide which is driving the demand for effective treatment options. Additionally, ongoing research and development activities for novel biologic therapies to treat lupus is expected to provide opportunities for market growth. Many pharmaceutical companies are making substantial investments in developing advanced monoclonal antibodies and other targeted drugs to address unmet needs in lupus treatment. However, high developmental costs of biologics combined with drug failure in late stages of clinical trials remains a challenge hampering the market growth to an extent.
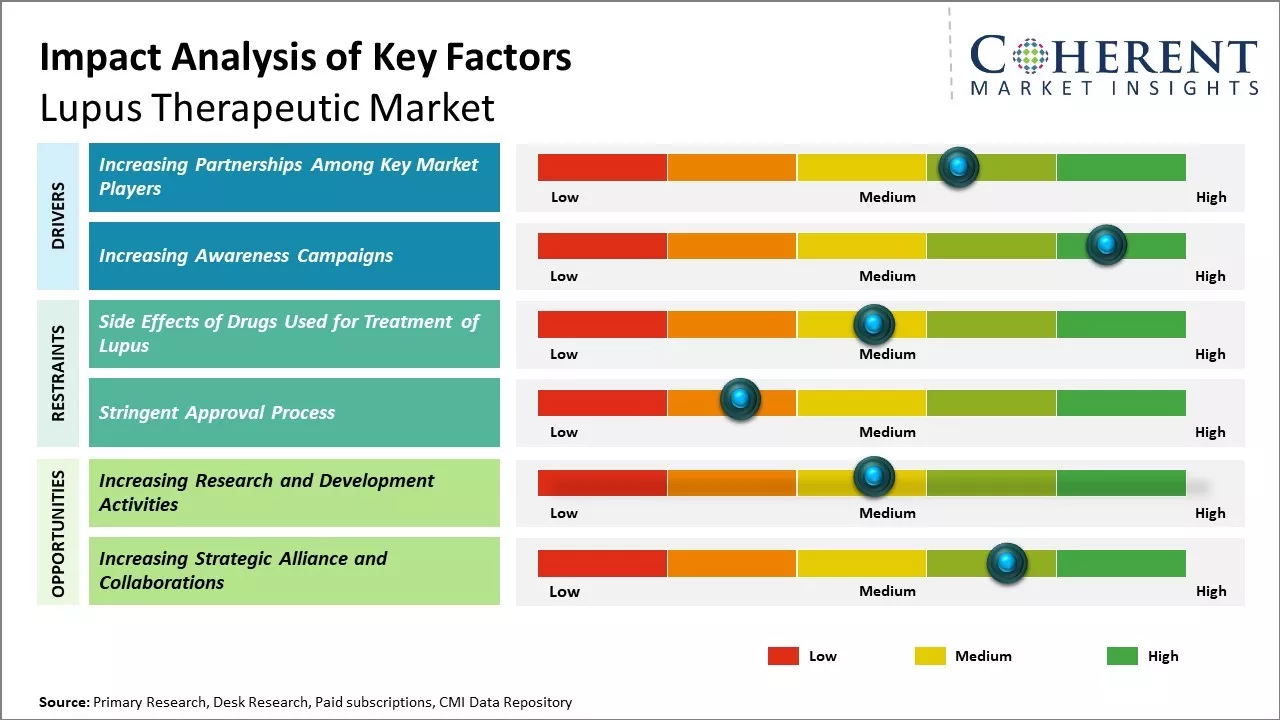
Increasing Partnerships Among Key Market Players
Increasing adoption of inorganic growth strategies, such as partnerships, is expected to drive the market growth. For instance, in March 2023, U.S. FDA’s Center for Drug Evaluation and Research partnered with the Lupus Research Alliance (LRA) to launch the Lupus Accelerating Breakthroughs Consortium (Lupus ABC), a first-of-its-kind public-private partnership focused on addressing challenges impacting lupus clinical trial success.
Get actionable strategies to beat competition: Download Free Sample
Increasing Awareness CampaignsIncreasing awareness campaigns is expected to drive the market growth over the forecast period. For instance, in October 2022, Aurinia Pharmaceuticals Inc., a global biotechnology company, announced that it had launched a digital campaign aimed at boosting awareness around a form of lupus that causes kidney disease, and it is not shying away from the tough nature of the condition. The campaign helps to show the “highly relatable, although sometimes uncomfortable” situations involved in the routine monitoring of lupus nephritis and highlights the importance of protecting the kidneys.
Key Takeaways from Analyst
The global lupus therapeutic market holds significant growth potential over the next decade. Lupus is a chronic autoimmune disease that disproportionately impacts women, presenting a growing unmet need. Increasing lupus disease prevalence globally, coupled with rising awareness and better diagnostics, will drive the demand for therapeutic options.
The market currently relies heavily on generic drugs like hydroxychloroquine, however patent expiries of major branded products will open the door for innovative biologics which offer targeted mechanisms ands improved safety profiles.
High development costs for novel lupus therapies pose a challenge as payers remain cautious to reimburse specialty drugs over generic alternatives. Demonstrating clear value benefits will be important for market uptake. Additionally, lupus being an inflammatory disease with diffuse symptoms makes it difficult to establish definitive efficacy endpoints in clinical trials. This increases regulatory hurdles for developers. Competitive pressures will also intensify as leading players seek to retain their footholds in the space through ongoing investments in R&D and business development deals.
Market Challenges: Side Effects of Drugs Used for Treatment of Lupus
The side effects of drugs used for the treatment of lupus is expected to hinder the growth of the global lupus therapeutic market over the forecast period. For instance, according to an article published by the Lupus Science and Medicine Journal (a peer-reviewed, open access journal) in March 2020, nearly all patients undergoing treatment for SLE report one or more adverse events such as nausea, vomiting, bone toxicity, and others.
Market Opportunities: Increasing Research and Development Activities
Increasing research and development activities among key market players is expected to offer lucrative growth opportunities over the forecast period. For instance, in November 2021, AstraZeneca, a U.K.-based biopharmaceutical company, announced positive results from its TULIP Phase 3 clinical trial program (focused on determining the efficacy and safety of Anifrolumab), stating that Saphnelo (anifrolumab) - a human monoclonal antibody showed greater reduction of SLE when used in combination with standard therapy than the use of only standard therapy.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
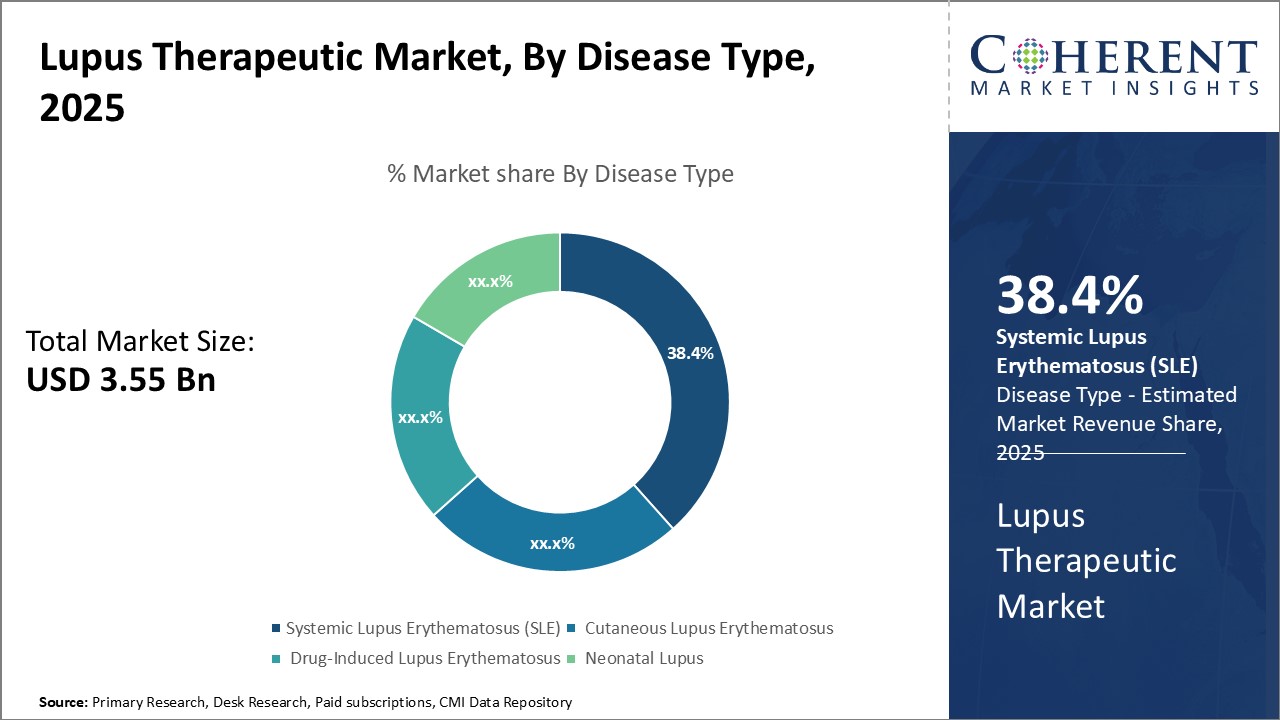
Insights, By Disease Type: Growing Prevalence of SLE Drives the Systemic Lupus Erythematosus SegmentDisease type segment is sub-segmented into systemic lupus erythematosus (SLE), cutaneous lupus erythematosus, drug-induced lupus erythematosus, and neonatal lupus. The alcohol addiction sub-segment is estimated to hold 38.4% of the market share in 2025, owing to its growing prevalence across the world. SLE is the most common form of lupus that can damage any part of the body including joints, skin, blood cells, lungs, heart and kidneys. The disease is nine times more common in women as compared to men and is known to flare up unexpectedly. It is estimated that SLE affects about 5 million people worldwide with around 1.5 million new cases each year. The prevalence of SLE has been on the rise especially in developing countries owing to growing environmental pollution, lack of early diagnosis and limited awareness. Besides its autoimmune nature, family history, certain medications and viruses are also known triggers for SLE. With no known cure available as of now, lifelong treatment and management of the disease continues to drive the demand for various SLE therapeutic drugs and biologics. The unpredictable nature of SLE flare ups also contributes to improved medication adherence among patients.
Insights, By Treatment Type: Growing Adoption of Biologics and Biosimilars Fuels the Non-Steroidal Anti-Inflammatory Drugs Segment
Treatment type segment is sub-segmented into non-steroidal anti-inflammatory drugs (NSAIDS), biologics, antimalarial drugs, corticosteroids, and others. The non-steroidal anti-inflammatory drugs (NSAIDS) sub-segment is estimated to hold 28.6% of the market share in 2025, owing to the growing adoption of biologics and biosimilars. NSAIDS have been conventionally used as the first line treatment for relieving joint pains, fever, and swelling associated with SLE. However, as the understanding of autoimmune pathways improved, biologics specifically targeting B-cells, T-cells, and other cytokine pathways have replaced conventional NSAIDS for SLE patients with severe organ involvement or those inadequate responsive to NSAIDS. Innovation in the field of biologics has led to the approval of several autoimmune pathway inhibitors like belimumab, rituximab, hydroxychloroquine, and mycophenolate over the past decade. Additionally, patent expiration of blockbuster biologics has also fueled the market entry of their cheaper biosimilar counterparts. This shift towards biologics from NSAIDS continues to positively impact the overall growth of the NSAIDS market segment.
Insights, By Distribution Channel: Ease of Access Boosts Hospital Pharmacy's Role in Treatment
Distribution channel segment is sub-segmented into hospital pharmacies, medical stores, and others. The hospital pharmacies sub-segment is estimated to hold 47.9% of the market share in 2025, owing to their concentrated market presence. As SLE is a complex multi-organ disease requiring continuous monitoring, most doctors prefer prescribing medications for SLE patients from hospital settings over retail stores. Hospital pharmacies employ qualified personnel who are trained to counsel patients on complex dosing schedules, drug interactions, side effects monitoring, and correct administration techniques for biologics and injectables that commonly feature in SLE treatment regimens. Moreover, hospitals also equip themselves with advanced pharmacy information systems that ensure proper tracking of prescriptions, inventory management and timely refill reminders. This enhances medication adherence, disease management and follow ups for SLE patients compared to other channels. In many countries, predominant health insurances also cover most or all hospital pharmacy costs, making it highly affordable for patients compared to other channels. With majority of SLE patients requiring long term care and multidisciplinary treatment involving rheumatologists, nephrologists and other specialists, concentration of these medical services in hospitals further propels the growth of hospital pharmacies segment.
Need a Different Region or Segment? Download Free Sample
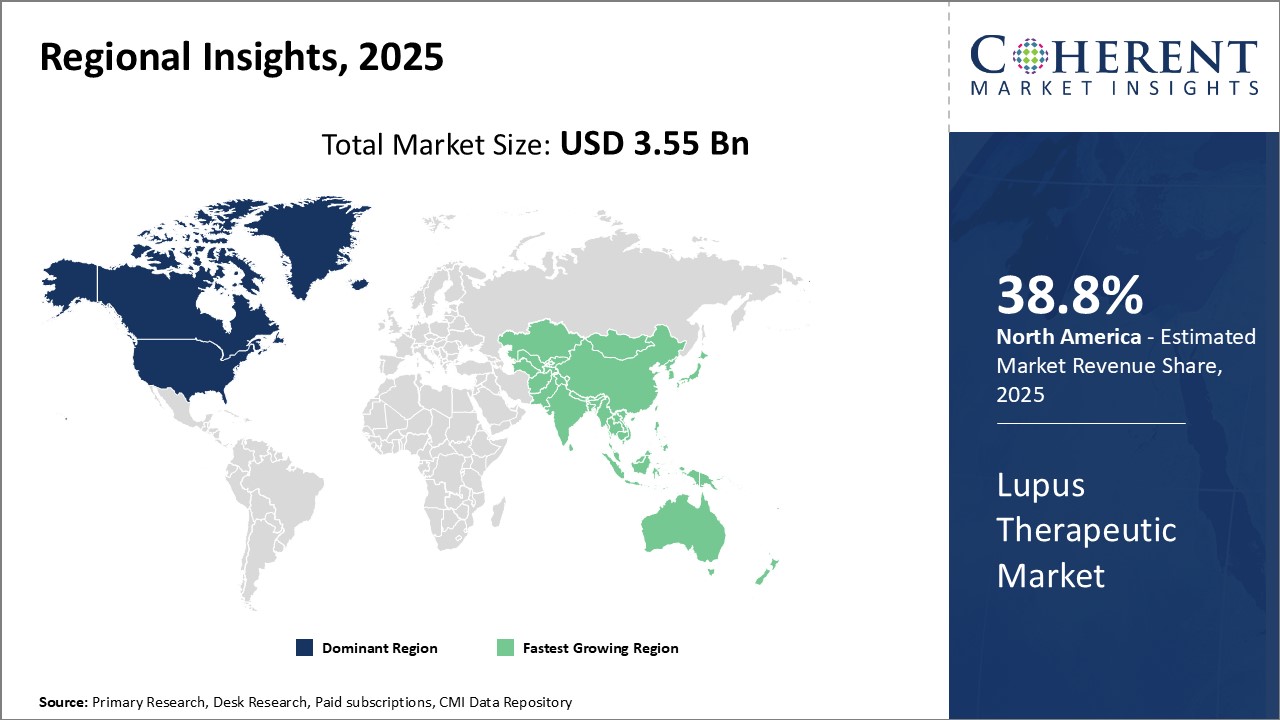
North America is the dominant region in the global lupus therapeutic market and is estimated to hold 38.8% of the market share in 2025. This can be attributed to the high prevalence rate of the disease in the region coupled with a strong presence of major players. The region is an early adopter of novel therapies and advanced treatment options leading to higher drug penetration. Moreover, favorable reimbursement policies have ensured the wider access and affordability of medications. It offers a promising growth opportunity owing to growing patient awareness initiatives by advocacy groups and rising healthcare expenditures over time. Developed healthcare infrastructure and focus of players to strengthen the regional market foothold through continuous R&D investments further supplements the market growth.
The Asia Pacific region is the fastest growing regional market for lupus therapeutics due to increasing incidence of lupus cases associated with growing geriatric populace in countries like China and India. Rapid economic development across emerging Asian nations has prompted improvements in healthcare access and services. This has positively impacted early diagnosis and treatment-seeking rate for lupus over the years. Rising patient affordability, availability of affordable generic medications, and influx of international brands have enhanced the affordability of treatments in Asia Pacific. Governments of various countries are focused on strengthening the biopharmaceutical industry by offering favorable foreign investment policies and funding. This is drawing the attention of global players to tap immense potential offered by the Asian market. Leading domestic firms are also striving to introduce novel forms of therapies for the better management of the disease. The growing regional pharmaceutical industry represents a key growth driver that is estimated to fuel the Asia Pacific lupus therapeutics market during the forecast timeline.
Lupus Therapeutic Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 3.55 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.1% | 2032 Value Projection: | USD 6.53 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
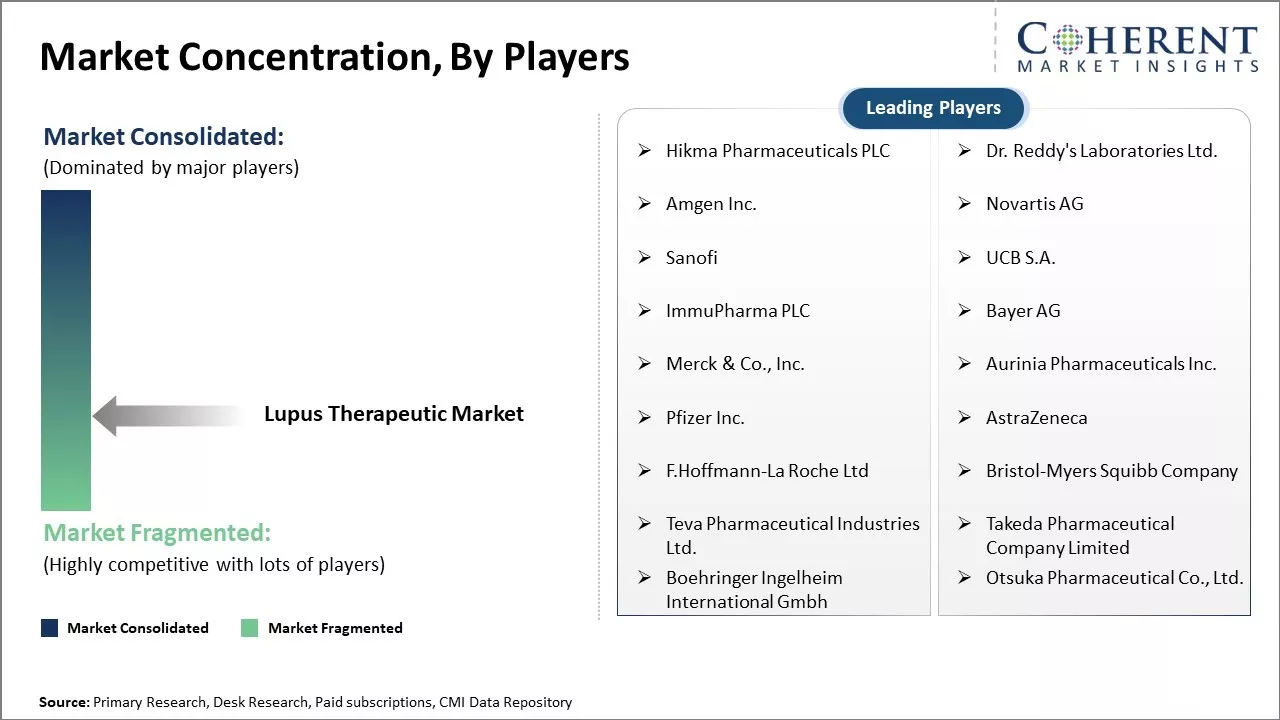
Hikma Pharmaceuticals PLC, Dr. Reddy's Laboratories Ltd., Amgen Inc., Novartis AG, Sanofi, UCB S.A., ImmuPharma PLC, Bayer AG, Merck & Co., Inc., Aurinia Pharmaceuticals Inc., Pfizer Inc., AstraZeneca, F.Hoffmann-La Roche Ltd, Bristol-Myers Squibb Company, Teva Pharmaceutical Industries Ltd., Takeda Pharmaceutical Company Limited, Boehringer Ingelheim International Gmbh, and Otsuka Pharmaceutical Co., Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: Lupus is a chronic autoimmune disease wherein the body’s immune system fails to recognize its cells and tissues as self and starts attacking them. The four major types of lupus include systemic lupus erythematosus (SLE), cutaneous lupus erythematosus, drug-induced lupus erythematosus, and neonatal lupus. Systemic lupus erythematosus is the most common type of lupus that causes damage to several organs throughout the body such as the kidney, lungs, heart, and others. Systemic lupus erythematosus causes inflammation in the skin, joints, lungs, blood cells, kidneys, etc.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients