The global acinetobacter pneumonia therapeutics market size is expected to reach US$ 1,076.0 Mn by 2032, from US$ 725.1 Mn in 2025, exhibiting a CAGR of 5.8% during the forecast period.
Acinetobacter pneumonia is an opportunistic infection caused by the Acinetobacter bacterium. These bacteria can cause a variety of healthcare-associated infections and are emerging as important pathogens globally. Currently, there are limited therapeutic options available for treating Acinetobacter pneumonia.
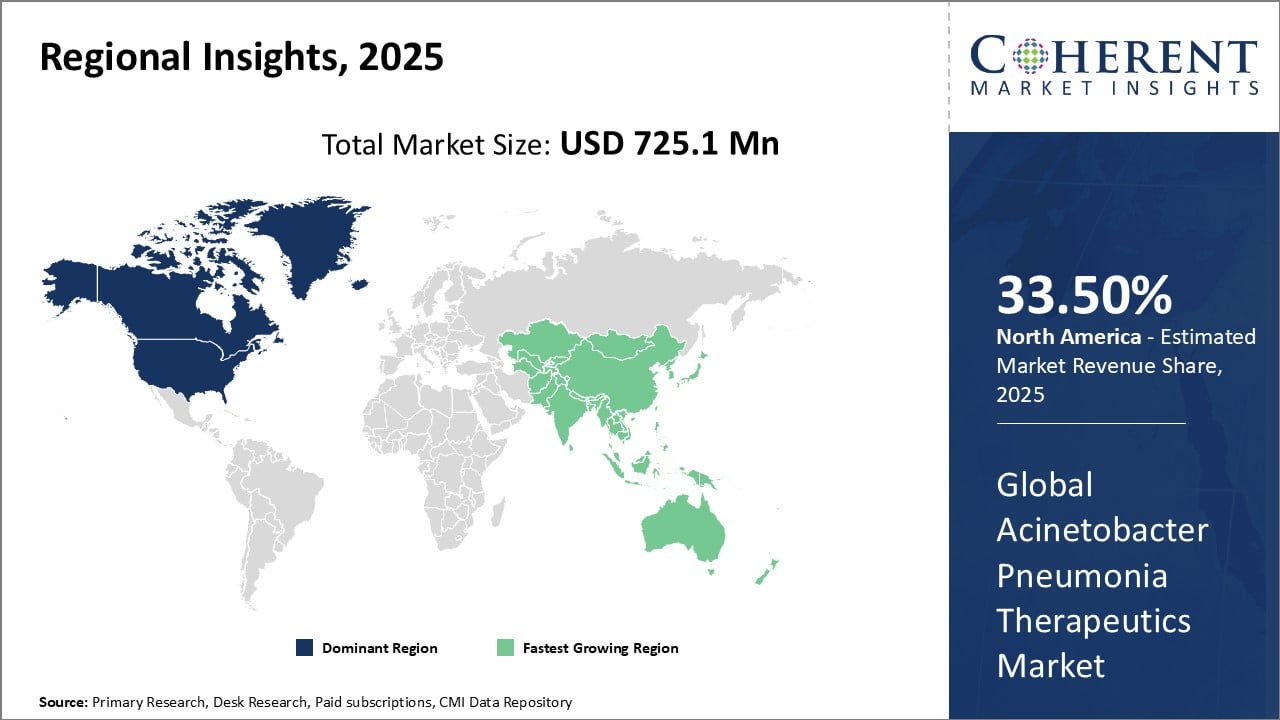
Global Acinetobacter Pneumonia Therapeutics Market- Regional Insights
- North America is expected to be the largest market for acinetobacter pneumonia therapeutics during the forecast period, accounting for over 35.5% of the market share in 2025. North America has dominated the Acinetobacter pneumonia therapeutics market for several years owing to a strong presence of leading pharmaceutical companies and advanced healthcare infrastructure in the region. Countries like the U.S. and Canada have witnessed high prevalence of pneumonia caused by Acinetobacter bacteria. This has driven significant research activities towards the development of novel therapeutics. Furthermore, favorable government policies have supported pharmaceutical manufacturers in commercializing innovative drugs. Rising healthcare spending in the region has enabled affordable access to diagnostic tests and therapies for pneumonia patients.
- Asia Pacific is expected to be the second-largest market for acinetobacter pneumonia therapeutics, accounting for over 30.1% of the market share in 2025. The Asia Pacific region has emerged as the fastest growing market for Acinetobacter pneumonia therapeutics in recent times. Surging instances of hospital-acquired and ventilator-associated pneumonia cases among critical patients in countries like China, India, and Japan are the major factors stimulating demand. The growing medical tourism industry has further augmented the availability of diagnostic and treatment options. Regional governments are also encouraging foreign investments and partnerships between global drug makers and local players to strengthen domestic production capabilities. This has played a vital role in improving supply chain and drive down costs of therapies.
- Europe has promising market with a share of 21.9% during the forecast period. The Acinetobacter pneumonia therapeutics market in Europe is witnessing growth due to the rising incidence of Acinetobacter infections, particularly in healthcare settings. Increased antibiotic resistance poses a significant challenge, necessitating advanced therapeutic approaches. The market is driven by ongoing research and development activities, collaborations, and strategic partnerships among pharmaceutical companies. Key players are focusing on novel drug formulations and treatment modalities to address the evolving resistance patterns. Government initiatives and healthcare awareness campaigns further contribute to market expansion. With a strong emphasis on innovation, the Acinetobacter pneumonia therapeutics market in Europe is poised for continuous advancement in the coming years.
Analyst’s Views:
The Acinetobacter pneumonia therapeutics market is poised to see steady growth over the coming years. Growing prevalence of drug-resistant infections along with rising cases of hospital-acquired pneumonia are some of the key drivers propelling the demand for novel treatment options. However, lack of approved therapeutics continues to be a challenge, with antibiotics being the primary treatment choice.
North America currently dominates the market and is expected to maintain its lead position. This can be attributed to growing R&D investments by pharmaceutical companies and rising healthcare spending in the region. At the same time, Asia Pacific is likely to witness highest growth during the forecast period. This can be credited to the improving healthcare infrastructure and increasing discretionary spending on medical care within emerging economies.
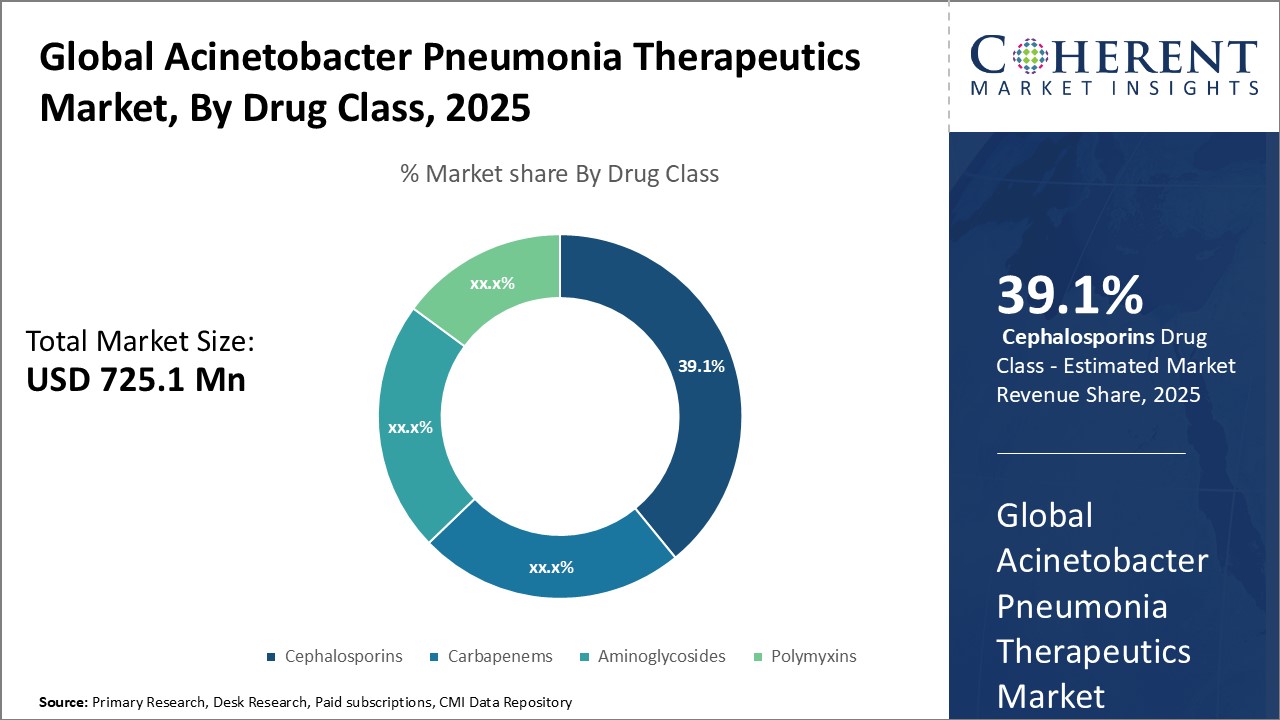
On the product side, antibacterial drugs hold the largest share currently. However, the development of new therapeutic classes such as small molecule immunomodulators and bacterial lysins are expected to gain traction going forward. With research shifting towards new targets and mechanisms of action to overcome antimicrobial resistance, these novel therapies are likely to transform the treatment landscape in the long run. Collaborations between public and private players have also increased for streamlining clinical development and expanding geographical reach.
Figure 1. Global Acinetobacter Pneumonia Therapeutics Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
Global Acinetobacter Pneumonia Therapeutics Market- Drivers
- Rising prevalence of Acinetobacter pneumonia infections: The rising prevalence of Acinetobacter pneumonia infections across healthcare settings is a key factor driving the growth of the Acinetobacter pneumonia therapeutics market. Acinetobacter pneumonia is considered as one of the most problematic nosocomial infections due to increasing antibiotic resistance. According to the Centers for Disease Control and Prevention (CDC), Acinetobacter causes approximately 8%–10% of all hospital-acquired pneumonias in the U.S. with mortality rates ranging from 26% to 65% (CDC, 2022). The increasing prevalence can be attributed to the rising elderly population that are more susceptible to pneumonia and growing antibiotic resistance. Most strains of Acinetobacter have become resistant to multiple classes of antibiotics used for treatment, including carbapenems, which were used as a last resort.
- Increasing research & development activities: Increasing research & development activities is one of the major factors driving the growth of the Acinetobacter pneumonia therapeutics market. Acinetobacter pneumonia is a significant cause of morbidity and mortality in hospitals worldwide. However, there are currently no approved vaccines or antiviral drugs available for the treatment of this multidrug-resistant pathogen. Due to the increasing prevalence of antibiotic-resistant Acinetobacter infections, there is a pressing need to develop new treatment options. Currently, several pharmaceutical and biotech companies have intensified their efforts to address this urgent medical need through extensive research. For example, Entasis Therapeutics, an advanced late-stage clinical biopharmaceutical company is developing a novel antibiotic called zoliflodacin specially designed to treat multidrug-resistant Acinetobacter infections. Phase 3 clinical trials of zoliflodacin started in 2021 and the drug showed promising results in treating patients with Acinetobacter pneumonia. Similarly, other companies such as Lefamulin by Pleiconcell, TP-271 and TP-6076 by Tetraphase Pharmaceuticals are in active clinical trial phases for treating multidrug-resistant Acinetobacter infections.
- Growing healthcare expenditure: Growing healthcare expenditure is one of the major factors driving the growth of the Acinetobacter pneumonia therapeutics market. Pneumonia caused by Acinetobacter bacteria has been rising steadily over the past decade posing severe threat. As per data from World Health Organization (WHO), the global incidence of healthcare associated pneumonia caused by multidrug resistant Acinetobacter bacteria increased by over 15% from 2015-2020. This rising caseload has placed significant economic burden on governments and healthcare systems worldwide. The high treatment cost of Acinetobacter pneumonia is further escalating the healthcare expenditure. Limited treatment options and growing antibiotic resistance has made the treatment lengthy and costly. For instance, a study published in American Journal of Infection Control in 2021 reported that the mean hospital cost per patient for healthcare associated drug resistant Acinetobacter Pneumonia in the U.S. was estimated to be over US$51,000. Considering millions of new cases being reported annually, the total expenditure on treatment runs into billions of dollars. This rising financial burden is forcing governments as well as private healthcare payers to focus on development of new and more effective therapeutics to curb the escalating costs.
Global Acinetobacter Pneumonia Therapeutics Market- Opportunities
- Emerging markets in developing countries: Emerging markets in developing countries present a great opportunity for growth in the Acinetobacter pneumonia therapeutics market. These developing nations are experiencing rapid economic development and population growth. With rising incomes, healthcare infrastructure and awareness, more patients now have access to diagnosis and treatment options for drug-resistant infections like Acinetobacter pneumonia. According to WHO data from 2021, lower respiratory infections remain one of the top ten causes of death in developing countries. Pneumonia alone was responsible for over 800,000 deaths in children under 5 years in 2020. Drug-resistant pathogens have further complicated treatment with limited affordable options. With a growing vulnerable patient population and a dearth of effective therapies, the need for innovative pneumonia treatments is huge in these emerging healthcare markets.
- Increased emphasis on preventive healthcare: Increased emphasis on preventive healthcare presents a major opportunity for the Acinetobacter pneumonia therapeutics market. As awareness grows about the importance of prevention over treatment, demand is rising for interventions that can help dodge this disease. Public health officials globally recognize the potential savings from shifting resources upstream. For example, the World Health Organization estimates US$1 invested in prevention could yield US$3 in reduced healthcare costs and lost productivity down the road. Pharmaceutical firms investing now in research and development of vaccines as well as rapid point-of-care diagnostics for early detection may gain first-mover advantage. By 2030, preventive solutions could account for over 30% of the therapeutics market according to some projections, up from under 20% currently. With coordinated global efforts, the future appears bright for curbing the widespread impact of this pneumonia through affordable prevention.
Global Acinetobacter Pneumonia Therapeutics Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 725.1 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.8% | 2032 Value Projection: | USD 1,076.0 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Pfizer Inc., Merck & Co., Inc., GlaxoSmithKline plc (GSK), AstraZeneca plc, Novartis AG, Johnson & Johnson, Sanofi S.A., Bayer AG, and Basilea Pharmaceutical |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Acinetobacter Pneumonia Therapeutics Market- Trends
- Advancements in molecular diagnostic techniques: Advancements in molecular diagnostic techniques are having a major impact on the Acinetobacter pneumonia therapeutics market. New polymerase chain reaction (PCR) based technologies allow for rapid and accurate diagnosis of Acinetobacter infections at the point-of-care. This provides clinicians with valuable information to prescribe appropriate targeted treatment options within the first 24-48 hours, leading to better patient outcomes.
- Rising preference for targeted drug therapy: The rising preference for targeted drug therapy is having a strong influence on the Acinetobacter pneumonia therapeutics market. With advancements in medical research, clinicians now have a better understanding of the mechanisms by which infectious agents cause disease. This is enabling them to develop more targeted treatment approaches that precisely attack vulnerable aspects of the infecting bacteria. Targeted therapies aim antibiotics and other drugs at specific components of the Acinetobacter bacteria that are crucial for its growth and survival. By disarming these strategic targets, these therapies can often achieve cure with lower doses or shorter durations of treatment compared to conventional broad-spectrum antibiotics. This spares patients from unnecessary exposure to high drug levels and helps prevent the further development of bacterial resistance. Pharmaceutical companies are increasingly focusing their research and development efforts on generating targeted drugs with novel mechanisms of action against Acinetobacter pneumonia.
Global Acinetobacter Pneumonia Therapeutics Market - Restraints
- High R&D costs: High research and development costs are certainly one of the major restraints for the growth of the Acinetobacter pneumonia therapeutics market. Developing new and effective drugs to treat such deadly infections is an extremely long, complex and costly process. According to the WHO data, on an average it takes over 10 years for any drug to move from initial discovery to final approval and commercial launch. During this period, the R&D costs continue to mount due to multiple stages of pre-clinical and clinical testing involved to prove safety and efficacy of the candidate drug. Most drugs fail or get dropped during clinical trials itself due to lack of efficacy or safety issues. This means all the investments made into the failed candidates become fruitless expenditures for the companies.
- Stringent regulatory guidelines: Stringent regulatory guidelines imposed by healthcare authorities across the globe are posing significant challenges for the growth of the Acinetobacter pneumonia therapeutics market. Developing effective drugs to treat infections caused by Acinetobacter bacteria is immensely complex and time-consuming due to the inherent drug-resistant nature and constantly evolving genetics of the bacteria. Pharmaceutical companies need to overcome numerous regulatory hurdles and comply with lengthy clinical trial phases to demonstrate safety and efficacy of new drug candidates as per the standards set by regulatory bodies like U.S. FDA and European Medicines Agency (EMA). Even after successful trials, the approval process takes additional 1-2 years of scrutiny of manufacturing and quality systems. This prolonged development cycle significantly increases the costs and financial risks for companies, discouraging investments in antibacterial drug research.
Figure 2. Global Acinetobacter Pneumonia Therapeutics Market Share (%), By Drug Class, 2025
To learn more about this report, Download Free Sample
Global Acinetobacter Pneumonia Therapeutics Market- Recent Developments
Business Development Activities by the Market Players
- On May 23, 2023, the U.S. Food and Drug Administration approved Xacduro (sulbactam for injection; durlobactam for injection), a new treatment for hospital-acquired bacterial pneumonia (HABP) and ventilator-associated bacterial pneumonia (VABP) caused by susceptible strains of bacteria called Acinetobacter baumannii-calcoaceticus complex, for patients 18 years of age and older
- On April 17, 2023, Innoviva, Inc., a diversified holding company with a portfolio of royalties and other healthcare assets, announced that the U.S. Food and Drug Administration’s (FDA) Antimicrobial Drugs Advisory Committee (AMDAC) unanimously voted 12-0 in support of approval based on a favorable benefit-risk assessment of sulbactam-durlobactam for the treatment of adults with hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia caused by susceptible strains of Acinetobacter baumannii-calcoaceticus complex (Acinetobacter)
Top Companies in the Global Acinetobacter Pneumonia Therapeutics Market
- Pfizer Inc.
- Merck & Co., Inc.
- GlaxoSmithKline plc (GSK)
- AstraZeneca plc
- Novartis AG
- Johnson & Johnson
- Sanofi S.A.
- Bayer AG
- Basilea Pharmaceutica
Definition:
Acinetobacter pneumonia is a respiratory infection caused by bacteria belonging to the Acinetobacter genus, particularly Acinetobacter baumannii. These bacteria are opportunistic pathogens often associated with healthcare settings, posing a significant threat to individuals with compromised immune systems. Acinetobacter pneumonia is characterized by symptoms such as fever, difficulty breathing, and coughing. The infection is challenging to treat due to increasing antibiotic resistance in Acinetobacter strains. Prevention and control measures, including strict hygiene practices in healthcare facilities, are crucial to managing the spread of Acinetobacter pneumonia, and ongoing research aims to develop effective therapeutics against this resilient bacterial infection.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients




