Hypertriglyceridemia Therapeutics Market is estimated to be valued at USD 12.19 Bn in 2025 and is expected to reach USD 17.15 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 5% from 2025 to 2032.
Analysts’ Views on Global Hypertriglyceridemia Therapeutics Market:
Over the forecast period, the global hypertriglyceridemia therapeutics market is anticipated to rise due to the rising prevalence of cardiovascular diseases (CVD) such atherosclerosis, hypertension, and coronary heart disease and sedentary lifestyle. For instance, on March 16 2023, according to the World Health Organization (WHO), an estimated 1.28 billion adults aged 30–79 years worldwide have hypertension, most (two-thirds) living in low- and middle-income countries such as Afghanistan, Haiti, Ghana, etc. An estimated 46% of adults with hypertension are unaware that they have the condition and a less than half of adults (42%) with hypertension are diagnosed and treated.
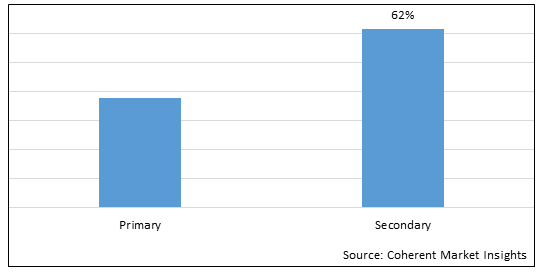
Figure 1. Global Hypertriglyceridemia Therapeutics Market Share (%), By Type, 2025
To learn more about this report, Download Free Sample
Global Hypertriglyceridemia Therapeutics Market– Drivers
Increase in sedentary lifestyle
Increasing sedentary lifestyle is expected to drive the growth of global hypertriglyceridemia therapeutics market over the forecast period. For instance, in January 2022, according to the Forbes, 25% of U.S. adults are physically inactive according to the study released by the Centers for Disease Control and Prevention (CDC), Puerto Rico adults in U.S had the highest level of inactivity at 49.4%, with Colorado the lowest at 17.7%.
Increasing product approval by the key players
Increasing product approval by the market key players is expected to drive the global hypertriglyceridemia therapeutics market over the forecast period. For instance, in April 2020, Dr. Reddy’s Laboratories Ltd., an India-based multinational pharmaceutical, launched Fenofibrate Tablets United States Pharmacopeia, a therapeutic equivalent generic version of Tricor (fenofibrate) Tablets, approved by the U.S. Food and Drug Administration (USFDA). Dr. Reddy’s Fenofibrate Tablets USP are available in 54 mg doses in bottle count sizes of 90 and 160 mg doses in bottle count sizes of 90 and 500.
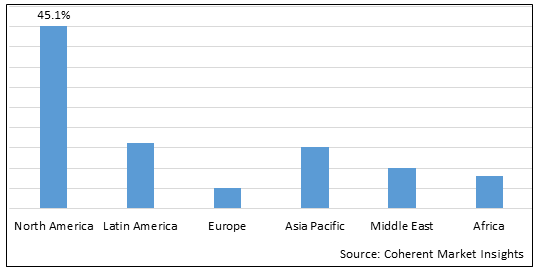
Figure 2. Global Hypertriglyceridemia Therapeutics Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
Global Hypertriglyceridemia Therapeutics Market- Regional Analysis
Among region, North America is estimated to hold a dominant position in the global hypertriglyceridemia therapeutics market over the forecast period owing to the increase prevalence of CVD such as coronary artery disease and atherosclerosis. For instance, on May 15, 2025, according to the CDC in U.S, coronary heart disease (CHD) is the most common type of heart disease, affecting about 375,476 people in 2021. About 1 in 20 adults age 20 and older have CAD (about 5%) and about 2 in 10 deaths from CAD happen in adults less than 65 years old.
Global Hypertriglyceridemia Therapeutics Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others faced problems regarding transportation of drugs from one place to another.
However, the COVID-19 pandemic had a negative impact on the global hypertriglyceridemia therapeutics market to the decrease in the supply chain of distribution of drugs due to lockdown. One of the most common conditions among healthy people, dyslipidemia affects between 30% and 60% of the population. As a result, it may be one of the comorbidities that COVID-19 patients experience the most frequently. For instance, in November 2022, according to a study published in the Frontiers, people with higher body mass index (BMI) also tend to be more vulnerable to SARS-CoV-2 infections. The risk and severity of COVID-19 have been frequently linked to dyslipidemia, and a statin is one of the basic treatments for individuals with dyslipidemia.
Hypertriglyceridemia Therapeutics Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 12.19 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5% | 2032 Value Projection: | USD 17.15 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Sanofi, GSK, plc., Biocon, Novo Nordisk A/S, Tonghua Dongbao Pharmaceutical Co., Ltd., Oramed, AbbVie Inc., Merck & Co., Inc. WOCKHARDT, Pfizer Inc., Julphar, Eli Lilly and Company, Bristol-Myers Squibb Company, ADOCIA, Hikma Pharmaceuticals PLC, Lupin, Zydus Pharmaceuticals, Inc., Glenmark Pharmaceuticals Ltd., Amneal Pharmaceuticals LLC., Aurobindo Pharma, and Accord Healthcare. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Hypertriglyceridemia Therapeutics Market- Segmentation
The global hypertriglyceridemia therapeutics market report is segmented into type, drug class, distribution channel, and region.
Based on Type, the global hypertriglyceridemia therapeutics market is segmented into primary and secondary. Out of which, the secondary segment is expected to dominate the global hypertriglyceridemia therapeutics market during the forecast period and this is this is attributed due to the unhealthy lifestyle factors and acquired medical issues caused due to the unhealthy lifestyle. So, the key players are focusing on strategies such as product launch for the global hypertriglyceridemia therapeutics market growth.
Based on Drug Class, the global hypertriglyceridemia therapeutics market is segmented into statins, fibrates, omega-3 fatty acids, HMG-CoA Reductase inhibitors, and niacin. The statins segment is expected to dominate the market over the forecast period and this is because there is such a high demand for them due to the fact that they reduce the body's production of cholesterol and the higher risk of heart attack or stroke.
Based on Distribution Channel, the global hypertriglyceridemia therapeutics market is segmented into retail pharmacy, hospitals pharmacy, online pharmacy, and others. The hospitals pharmacy segment is expected to dominate the market over the forecast period and this is attributed to the increasing prevalence of cardiovascular diseases.
Based on Region, the global hypertriglyceridemia therapeutics market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa. Out of which, the North America is expected to dominate the market over the forecast period owing to the increase in launch of product by market key players.
Among all segmentation, the drug class segment has the highest potential due to the increasing product launch as well as partnership by the key market players over the forecast period. For instance, in December 2019, Amarin Pharmaceuticals Ireland Limited, a cardiovascular health pharmaceutical company received U.S FDA approval for VASCEPA (icosapent ethyl) indicated for reducing cardiovascular risk. The novel indication for VASCEPA is that it is an adjunct to diet to reduce triglyceride levels in older patients with severe hypertriglyceridemia.
Global Hypertriglyceridemia Therapeutics Market- Cross Sectional Analysis
Among type, the secondary segment held a dominant position in North America region over the forecast period due to increasing number of cardiovascular diseases such as coronary artery disease and atherosclerosis. For instance, in March 2021, according to an article published in the Journal of American Journal of Preventive Cardiology, prevalence of (Atherosclerotic cardiovascular disease) ASCVD in the U.S. in 2020 was 24.0 million, approximately 10% of the total U.S. population.
Global Hypertriglyceridemia Therapeutics Market- Key Developments
On March 22 2023, Regeneron Pharmaceuticals, Inc., a biotechnology company, announced the U.S. Food and Drug Administration (FDA) has extended the approval of Evkeeza (evinacumab-dgnb) as an adjunct to other lipid-lowering therapies to treat children aged 5 to 11 with homozygous familial hypercholesterolemia (HoFH). Evkeeza is the first angiopoietin-like 3 (ANGPTL3) inhibitor treatment indicated for children as young as 5 years old to control dangerously high levels of low-density lipoprotein cholesterol (LDL-C) caused by HoFH. Evkeeza was initially approved as an adjunct to other lipid-lowering therapies in those aged 12 years and older with HoFH.
In May 2022, APONTIS PHARMA Deutschland GmbH & Co. KG, a pharmaceutical company, specializing in Single Pills announced a new development cooperation with Develco Pharma Switzerland, a private company that develops and produces innovative formulations for drugs with known active ingredients. The object is the contract development of two Single Pills, each with a combination of two leading active ingredients for the treatment of cardiovascular diseases caused by the hyperlipidemia.
In January 2022, Pfizer Inc., a multinational pharmaceutical and biotechnology company, and Ionis Pharmaceuticals, a biotechnology company that specializes in discovering and developing RNA-targeted therapeutics, announced the discontinuation of the Pfizer-led clinical development program for vupanorsen (PF-07285557), an investigational antisense therapy that was being evaluated for potential indications in cardiovascular (CV) risk reduction and severe hypertriglyceridemia (SHTG).
In February 2021, Regeneron Pharmaceuticals, Inc., a biotechnology company, announced that the U.S. Food and Drug Administration (FDA) approved EvkeezaTM (evinacumab-dgnb) as an adjunct to other low-density lipoprotein cholesterol (LDL-C) lowering therapies to treat adult and pediatric patients aged 12 years and older with homozygous familial hypercholesterolemia (HoFH). Evkeeza is the first U.S. FDA-approved treatment that binds to and blocks the function of angiopoietin-like 3 (ANGPTL3), a protein that plays a key role in lipid metabolism.
Global Hypertriglyceridemia Therapeutics Market- Key Trends
Collaboration of market key players for the product development
Collaboration of market key players for the product development is also expected to drive the market over the forecast period. For instance, in September 2021, Novartis AG, a healthcare company that focuses on the discovery, development, manufacture and marketing of prescription and generic pharmaceutical products, had reached a commercial agreement with the (National Health Society) NHS in England as part of a collaboration to pioneer a first-of-its-kind population health management approach to address elevated (low-density lipoprotein-cholesterol) LDL-C in eligible patients with (atherosclerotic cardiovascular disease) ASCVD across England. The NHS and Novartis collaboration moves into the implementation phase following the positive final recommendation from the National Institute for Health and Care Excellence (NICE) for use of inclisiran in primary care for the treatment of adult patients within its licensed indication1 who also have persistently elevated LDL-C levels (2.6 mmol/l or more despite maximum tolerated statins with or without ezetimibe) and a history of certain cardiovascular events.
Global Hypertriglyceridemia Therapeutics Market: Restraints
High cost of the treatment
High cost of the treatment for the hyperlipidemia is expected to restrict the growth of global hypertriglyceridemia market over the forecast period. For instance, in February 2022, according to an article published by the Well Mark Inc., high cholesterol levels are linked to a higher risk of cardiovascular disease, diabetes, and high blood pressure. Heart disease is the leading cause of death in the U.S., and costs around US$ 207 billion annually in lost productivity and medical expenses. Moreover, according to the TalktoMira, Inc., an innovative healthcare company with a mission to bring affordable and immediate healthcare access, the average retail cost of statins used to treat moderate cases of high cholesterol is US$ 139.29 for generic medications and US $360.43 for brand-name statins. So, to avoid the high cost the use of omega-3-fatty acids as well as niacin can be preferred for the treatment.
Global Hypertriglyceridemia Therapeutics Market- Key Players
Major players operating in the global hypertriglyceridemia therapeutics market include Sanofi, GSK, plc., Biocon, Novo Nordisk A/S, Tonghua Dongbao Pharmaceutical Co., Ltd., Oramed, AbbVie Inc., Merck & Co., Inc. WOCKHARDT, Pfizer Inc., Julphar, Eli Lilly and Company, Bristol-Myers Squibb Company, ADOCIA, Hikma Pharmaceuticals PLC, Lupin, Zydus Pharmaceuticals, Inc., Glenmark Pharmaceuticals Ltd., Amneal Pharmaceuticals LLC., Aurobindo Pharma, and Accord Healthcare.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients