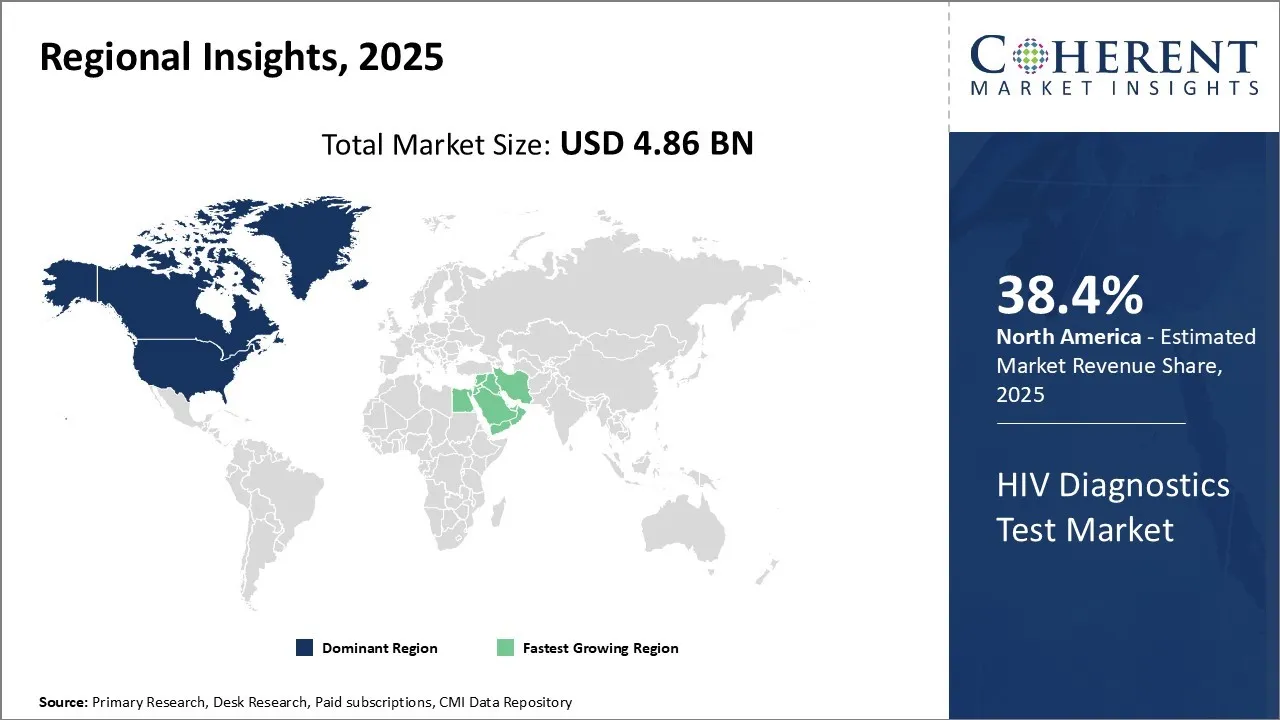
The HIV Diagnostics Test Market is estimated to be valued at USD 4.86 Bn in 2025 and is expected to reach USD 9.12 Bn by 2032, growing at a compound annual growth rate (CAGR) of 9.4% from 2025 to 2032.
To learn more about this report, Download Free Sample
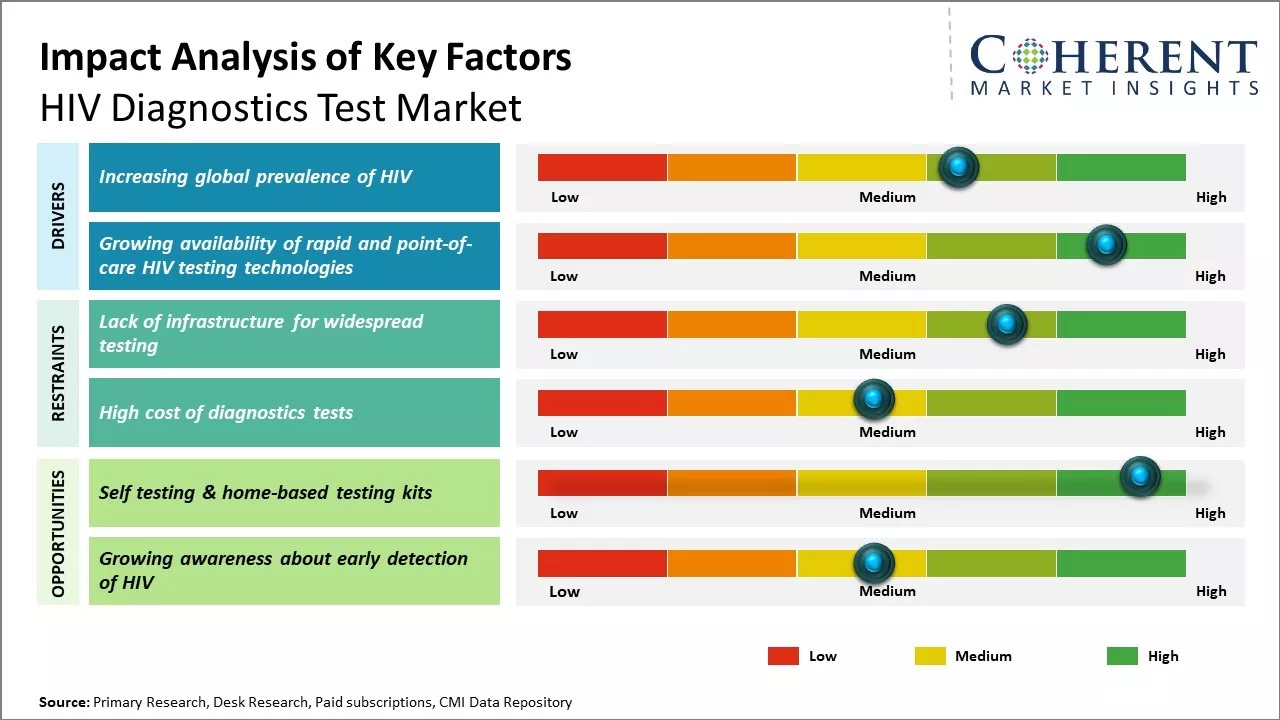
The market is expected to witness positive growth during the forecast period. Rapid expansion of healthcare investments and increasing awareness about HIV diagnosis are expected to boost market growth. In addition, rising initiatives by government and private organizations to spread awareness about HIV diagnosis and treatment is also expected to support the market growth during this period. However, costly diagnostics procedures and issues related to low accessibility in remote areas may hamper market growth.
|
Current Events |
Description and its impact |
|
Geopolitical conflicts and supply chain disruptions |
|
|
Technological advancements and innovation in diagnostics |
|
|
Socio-economic and demographic events |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The Centers for Disease Control and Prevention (CDC) funds initiatives like the Together TakeMeHome program, which provides free at-home HIV self-tests to U.S. residents aged 17 and older. Participants can order up to two free tests every three months until 2027, aiming to increase HIV awareness and early detection.
Medicaid covers routine HIV screening as a preventive service, though it is considered an optional benefit, and coverage varies by state in U.S. Private insurers may cover HIV tests for individuals with known or perceived risk factors, but policies differ among insurers.
Artificial Intelligence (AI) is significantly transforming the HIV diagnostics test market by enhancing accuracy, accessibility, and efficiency in detection and monitoring. AI facilitates rapid testing by integrating with biosensors and next-generation molecular diagnostics, such as CRISPR-based methods and loop-mediated isothermal amplification (LAMP). These technologies enable quicker and more cost-effective HIV detection, crucial for timely intervention. The Melbourne Sexual Health Centre's Artificial Intelligence and Modelling in Epidemiology Program has developed MySTIRisk, an AI-based web tool for assessing the risk of HIV and sexually transmitted infections.
The product segment includes assay kit and consumables. Assay kit segment is estimated to hold 59.7% of the market share in 2025. Assay kits have seen significant improvements that have made testing simpler, more convenient, and accessible. Traditionally, HIV testing required specialized laboratory equipment and trained healthcare workers to perform. However, assay kit manufacturers have miniaturized tests so they can be performed with simplified equipment right in a doctor's office or even in home settings. This decentralized testing removes barriers to access and promotes more frequent screening. Assay kit makers also focus on test design to eliminate complex multi-step procedures that might dissuade patients. Newer generations of kits employ streamlined, user-friendly formats like simple prick tests that produce results within minutes with only a single drop of blood. Such innovations boost compliance and catch infections earlier.
For instance, Thermo Fisher Scientific has introduced its Applied Biosystems HIV-1 Genotyping Kit with Integrase. This research-use-only assay analyzes HIV-positive samples to detect genetic variations that may confer resistance to widely used antiretroviral treatments.
The test type segment includes antibody test, antigen/antibody tests, and nucleic acid test. Antibody test contributes the highest share of the HIV diagnostics test market and is estimated to hold 44.1% of the market share in 2025. Being the first developed test type, antibody detection set the baseline standard of care in HIV screening. It identifies the body’s immune response to infection by detecting antibodies produced against HIV. While newer forms like antigen/antibody combinations and nucleic acid methods provide additional insight, no alternative has proven more sensitive and cost-effective for broad population screening. Guidelines continue recommending antibody tests as the frontline screening methodology. Their wide availability and regulatory approval across all regions and settings, from remote clinics to large reference labs, cemented antibody tests role as the globally preferred first-line assay. Antibody tests also offer distinct benefits like stability at varying storage temperatures required for resource-limited areas. Apart from this the rising demand for quick and accurate results is also propelling the demand for antibody test.
For instance, in March 2025, bioLytical introduced the 1-Minute INSTI® Multiplex HIV-1/2 Syphilis Antibody Test in Australia. This test provides highly accurate results in just 60 seconds using a single sample, making it the fastest dual HIV and syphilis rapid test available. This is further accelerating the HIV diagnostics test market demand.
The end user segment includes hospitals & clinics, diagnostic laboratories, homecare settings, and others. Diagnostic laboratories contribute the highest share of the HIV diagnostics test market and is estimated to hold 39.1% of the market share in 2025. Though hospitals initially managed most testing capacity, scaling screening require developing infrastructure beyond acute care settings. Diagnostic labs thus play a crucial role decentralizing testing closer to target populations. Their specialized expertise and regulatory compliance allow standardizing quality assessment across a network of customers. This provides health ministries and international programs an efficient, accountable partner for national screening cascades. By positioning multiple patient service centers, diagnostic labs facilitate walk-in access critical to identifying undiagnosed ‘key populations. Leveraging economies of scale in consumables procurement and coordination of supply chains keeps per-test costs low. This allows diagnostic networks offering subsidized or free panels to underinsured groups per public health priorities.
All India Institute of Medical Sciences (AIIMS) laboratory provides comprehensive diagnostic and monitoring services for HIV/AIDS patients. It was the first lab at AIIMS to receive NABL accreditation. Additionally, it plays a pivotal role in the HIV DBS sentinel surveillance program and serves as a training hub for technicians across India.
To learn more about this report, Download Free Sample
North America has remained the dominant region in the global HIV diagnostics test market over the past decade and is estimated to hold 38.4% of the market share in 2025. Strong presence of leading international players, high awareness levels regarding sexually transmitted infections (STI) diagnosis and favorable reimbursement policies have augmented the demand for HIV diagnostic tests in the country. In addition, the growing HIV awareness campaigns is also proliferating the HIV diagnostics market demand. In April 2025, Cook County Health launched a public health campaign to raise awareness of HIV prevention, testing, and services, targeting populations most at risk in Chicagoland.
The HIV diagnostics test market in the Africa region is anticipated to develop at the highest CAGR over the forecasted period attributed to the increasing healthcare investment by local and foreign organizations. The antibody test subsegment currently holds the prominent share in the HIV diagnostics test market in the Africa region. This is driven primarily by the high adoption of fourth generation antibody tests in the region over the past 2 years. Fourth generation antibody tests are now widely used for HIV screening across major healthcare facilities in South Africa, Nigeria and Ethiopia due to their ability to detect both anti-HIV antibodies and the HIV p24 antigen. This allows for a much earlier detection of the infection compared to previous generations of antibody tests. Several observational studies conducted by UNAIDS and country-specific health ministries have found that the increased use of fourth generation antibody tests has helped reduce new HIV infections in the region by making it possible to identify positive cases much sooner. This is further contributing to the HIV diagnostics market revenue.
The HIV diagnostics market in Canada is experiencing significant growth, driven by technological advancements, increased accessibility, and supportive public health initiatives. The demand for these products is fuelled by frequent testing requirements, particularly in high-risk populations. Apart from this, the escalating demand for accurate results is acting as one of the key factors propelling adoption of the HIV test. In January 2025, MedMira Inc. announced that Health Canada approved its Reveal® Rapid G4 HIV-1/2 Test for Point-of-Care use. With a sensitivity of 99.64% and specificity of 99.71%, the test provides fast, reliable, and high-quality results, making it ideal for healthcare professionals in any setting.
The HIV diagnostics market in India is experiencing significant growth, driven by technological advancements, increased accessibility, and supportive public health initiatives. Molbio Diagnostics has introduced a new RT-PCR test for the differential diagnosis of HIV-1 and HIV-2, providing results within 60 minutes. This test operates on the existing Truelab infrastructure, requiring no additional equipment or setup. Truenat, the platform supporting this test, is a portable, battery-operated, IoT-enabled Real-Time PCR system capable of diagnosing over 35 diseases. The introduction of this test aims to enhance early detection and viral load estimation, crucial for effective HIV management and monitoring, especially in resource-limited settings.
To learn more about this report, Download Free Sample
The global prevalence of HIV has been steadily rising over the past few decades posing a significant risk to public health across the world. UNAIDS estimates that approximately 38 million people were living with HIV globally at the end of 2020, with new infections and AIDS-related deaths remaining unacceptably high in many regions. Sub-Saharan Africa in particular has borne the brunt of the epidemic where around 68% of all people living with HIV reside. While new infections and deaths from AIDS have declined significantly in some countries due to treatment scale-up, many nations still face severe challenges in diagnosing those unaware of their HIV status and linking them to effective care. According to WHO, in 2020, there were an estimated 1.5 million new HIV infections and 680,000 AIDS-related deaths worldwide. This is further propelling the HIV diagnostics test industry.
While conventional HIV testing methods centered on laboratory-based immunoassays, recent years have witnessed significant advancements in rapid and point-of-care approaches. These innovative formats have played a transformative role in widening access to diagnostic services particularly in resource-constrained areas. Traditional testing involved sending blood samples to centralized laboratories for ELISA and Western blot analysis which could take days to provide results. This created barriers for timely diagnosis due to the need for multiple visits and loss to follow up. It was also challenging in remote communities with limited laboratory infrastructure.
Self-testing and home-based HIV testing kits provide a private and confidential way for individuals to know their status without having to visit overburdened testing centers or clinics. For instance, in December 2024, the Department of Health (DOH) unveiled the HIV self-test in the Cordillera region. This helps address a major barrier to testing which is the stigma still associated with HIV/AIDS. By making testing accessible in the privacy of one's home, more people especially from vulnerable groups will feel comfortable taking a test. With testing made easier, more frequent self-testing or self-screening at home would become the new norm. Campaigns by governments and NGOs promoting self-testing as the way forward are already raising awareness.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 4.86 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.4% | 2032 Value Projection: | USD 9.12 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
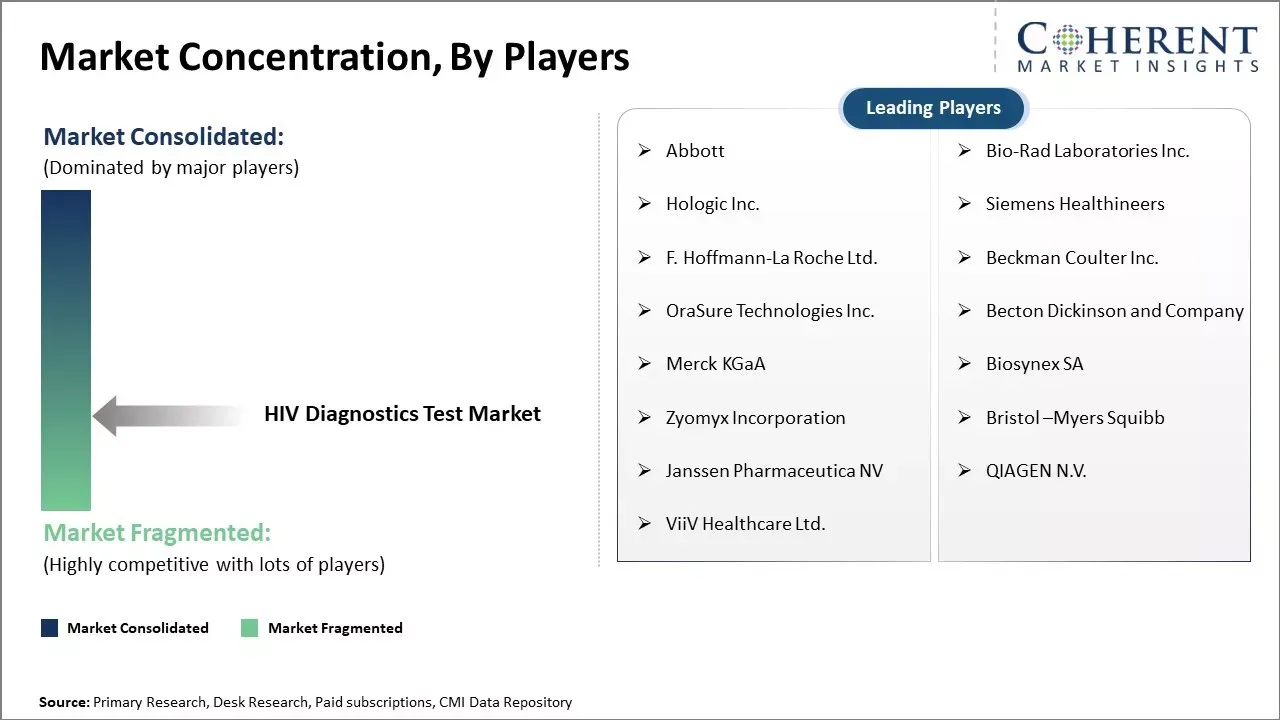
| Companies covered: |
Abbott, Bio-Rad Laboratories Inc., Hologic Inc., Siemens Healthineers, F. Hoffmann-La Roche Ltd., Beckman Coulter Inc., OraSure Technologies Inc., Becton Dickinson and Company, Merck KGaA, Biosynex SA, Zyomyx Incorporation, Bristol –Myers Squibb, Janssen Pharmaceutica NV, QIAGEN N.V., and ViiV Healthcare Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients