HIV diagnostics plays a major role in HIV prevention, treatment, care, and other support services. The diagnosis of HIV includes testing services in health-care facilities, free-standing sites, and a wide range of community-based approaches along with HIV self-testing. Developments such as home testing represent new and major opportunities for addressing HIV among men who have sex with men (MSM). According to the UNAIDS, the Joint United Nations Program on HIV and AIDS, 2018 data, Asia Pacific recorded around 5.2 million people living with HIV in 2022.
The Asia Pacific HIV Diagnostics Market is estimated to be valued at US$ 522.5 million in 2022 and is expected to exhibit a CAGR of 12.4% during the forecast period (2022-2030).
Figure 1. Asia Pacific HIV Diagnostics Market Share (%), by Product Type, 2022
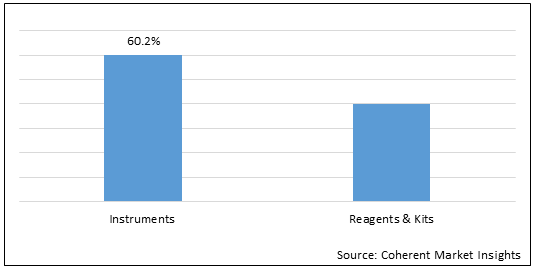
To learn more about this report, Download Free Sample
Increasing government initiatives to reduce HIV/AIDS burden are expected to drive Asia Pacific HIV diagnostics market over the forecast period
Governments of various countries in Asia Pacific are taking initiatives to create awareness regarding HIV among people due to high prevalence of HIV/AIDS in the region. This in turn is increasing adoption of HIV diagnostic instruments and test kits. Low literacy rate coupled with unawareness regarding transmission mode on HIV, changing lifestyle, and unsafe sex are some of the factors leading to increasing number of HIV cases. In order to lower the disease incidence and increase life expectancy of infected patients, governments of various countries along with NGOs are focusing on improving HIV diagnostics and making them easily accessible to the general population.
Furthermore, in April 2022, UNAIDS regional and country teams met with key government partners, civil society and community networks at the Regional DFAT Grant Implementation Meeting to share progress, achievements, opportunities and areas for strengthened collaboration in Cambodia, Indonesia, Papua New Guinea and the Philippines. The meeting took place in Bangkok, Thailand, in April 2022, and was joined by the government, civil society, communities and country office representatives from Cambodia, Indonesia, Papua New Guinea and the Philippines.
Moreover, in January 2022, Asian Development Bank (ADB) provided a strategic link between Myanmar’s government and the non-governmental organizations (NGOs) currently providing HIV/AIDS prevention and treatment services in order to strengthen the provision of healthcare in remote, vulnerable, and hard-to-reach populations.
Asia Pacific HIV Diagnostics Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 522.5 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 20 to 20 CAGR: | 12.4% | 2030 Value Projection: | US$ 1,333.4 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Abbott Laboratories, Merck KGaA, F. Hoffmann-La Roche AG, Atomo Diagnostics, Siemens Healthineers, Inc., bioLytical Laboratories Inc., MedMira Inc., Becton, Dickinson and Company, Bio-Rad Laboratories, Inc., and Danaher Corporation |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Figure 2. Asia Pacific HIV Diagnostics Market Share (%), by Region, 2022
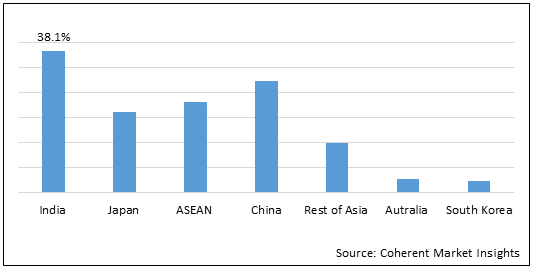
To learn more about this report, Download Free Sample
Introduction of the new diagnostic techniques to test HIV is expected to drive the global Asia Pacific HIV diagnostics market
Introduction of the new diagnostic techniques to test HIV is expected to drive the Asia Pacific HIV diagnostics market over the forecast period. For instance, in July 2018, according to the data published by the World Health Organization, the introduction of the in-vitro diagnostics (IVDs) tests has helped to diagnose patients effectively and work to provide appropriate treatments. In November 2018, in vitro diagnostic testing was published in the World Health Organization List (First WHO Model List of Essential In Vitro Diagnostics) for diagnosis of the HIV. These In Vitro (IVD) are grouped by discipline (e.g. clinical chemistry, serology, haematology, microbiology and mycology) and test type (e.g. bilirubin, complete blood count). In Vitro Diagnostics for the detection, diagnosis and monitoring of the WHO priority diseases: HIV infection, tuberculosis (TB), malaria, hepatitis B, hepatitis C, human papillomavirus (HPV) infection and syphilis. These IVDs are grouped by disease area and analyte tested.
Asia Pacific HIV Diagnostics Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe and the World Health Organization had declared it a public health emergency on January 30, 2020.
COVID-19 can affect the economy in three main ways: by directly affecting production and demand of drugs, medical devices, diagnostic testing by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, U.A.E., Egypt, and others, are facing problems with regards to the transportation of drugs from one place to another.
However, the COVID-19 pandemic had a negative impact on the Asia Pacific HIV diagnostics market, owing to the increased stress for testing COVID 19 Samples there was slight decrease in the diagnostics testing on HIV. For instance, according to the data published by the National Center for Biotechnology Information, due to the COVID-19 pandemic, significant disruptions in medical services occurred worldwide. In clinical experience, one change was reduced outpatient HIV testing. The research carried out by the PubMed (the MEDLINE database) search revealed reports documenting reduced HIV testing in Australia, Belgium, China, Japan, and multiple regions of Kenya, Uganda, and numerous other European countries. The COVID-19 pandemic period between January and August 2020 impacted significantly on HIV testing, the percentage of positive tests, the number of consultations and the number of new enrolments in care, despite the implementation of several mitigation strategies.
Asia Pacific HIV Diagnostics Market: Key Developments
In November 2021, the World Health Organization released new toolkit to enable countries to accelerate their ongoing efforts to fully adopt WHO guidelines and transition to new HIV testing algorithms. The guidance also highlighted the need to introduce dual HIV/syphilis rapid diagnostic tests (RDTs) and to phase out older testing technologies, including western blotting methods.
Asia Pacific HIV Diagnostics Market: Restraint
The major factors that hinder growth of the Asia Pacific HIV diagnostics market include inadequacy of competent healthcare personnel, unreliability of test results, and poor staff attitudes are the factors which stop patients from HIV screening. For instance, in June 2017, Health Sciences Authority (HSA), Singapore; recalled 9 lots of HIV screening test kits, after it was found that the kits could result in false negative results for some people in the early stages of HIV infection. Furthermore, lack of awareness regarding testing procedures, inaccessibility to testing sites, inconvenient testing hours, and cost of testing are also negatively impacting Asia Pacific HIV diagnostics market growth. Several variants of the virus are found worldwide and some variants tend to be more prevalent in certain geographies than others. Testing kits, when not designed to accommodate this variation, can provide incorrect results.
Key Players
Major players operating in the Asia Pacific Hiv diagnostics market include Abbott Laboratories, Merck KGaA, F. Hoffmann-La Roche AG, Atomo Diagnostics, Siemens Healthineers, Inc., bioLytical Laboratories Inc., MedMira Inc., Becton, Dickinson and Company, Bio-Rad Laboratories, Inc., and Danaher Corporation.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients