An allergy is a medical condition where the immune system reacts abnormally when exposed to certain foreign substances. These allergy-producing substances are known as allergens. Allergens can be dust particles, mites, molds, pollens, animal proteins, foods, and medications.
Allergies can be perennial or seasonal. Perennial allergies occur due to specific allergens that cause an allergic reaction throughout the year. Allergens responsible for perennial allergies include pet hair or dander, food, and medication. Seasonal allergies occur due to weather changes that allow growth of mold, insect or grass. Seasonal allergies are caused by airborne allergens such as pollens and ragweed. Symptoms of allergic reactions include itching, runny & blocked nose, watery eyes, wheezing, shortness of breath, swelling in lips, eyes or face, sneezing, and others.
Europe and Japan Allergy Immunotherapy Market - Impact of the Coronavirus (COVID-19) Pandemic
The COVID-19 pandemic is expected to limit growth of the Europe and Japan allergy immunotherapy market, owing to disruption of transportation between the countries. For instance, according to the European Medicines Agency (EMA): 2020, the European medicines regulatory network established the steering group in March 2020 in response to the COVID-19 pandemic. It consists of representatives of EMA, the European Commission, Heads of Medicines Agencies (HMA), and coordination groups for mutual recognition and decentralized procedures for human and veterinary medicines (CMDh and CMDv), as well as risk communication specialists. The steering group does the work of developing methods for the collection and sharing of data on demand for medicines across European Union (EU) and improve the forecasting of demand for medicines.
Due to COVID-19 pandemic, there has been increase in social distancing, maintenance of hygiene, etc. Moreover, practical recommendations have been developed for the improvement of care for allergic patients in daily routine. Also, telemedicine has aroused as a very valuable tool for handling allergic patients.
During the pandemic, the European Academy of Allergy and Clinical Immunology (EAACI) proposed several research papers and clinical recommendations for daily clinical care of allergic patients. One of the most important therapies in allergic patients is allergen immunotherapy (AIT) as the only disease-modifying treatment option in IgE-mediated allergic diseases. According to the guidelines updated by EAACI and the journal ‘Allergy and Its Impact on Asthma’ (ARIA-)initiative outlined practical recommendations on AIT, if COVID-19 is suspected or confirmed, all kinds of AIT should be temporally interrupted as a general rule in infectious diseases. Moreover, if the patient is free of symptoms without evidence of the disease, then sublingual immunotherapy (SLIT) can be administered at home supported by telemedicine. This option can help in maintaining adherence to treatment as well as in follow-up of allergic disease evolution and confirmation of absence of COVID-19.
The Europe and Japan allergy immunotherapy market is estimated to be valued at US$ 1,228.37 Mn in 2022 and is expected to exhibit a CAGR of 8.1% over the forecast period (2022-2030).
Europe and Japan Allergy Immunotherapy Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 1,228.37 Mn |
| Historical Data for: | 2018 to 2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 8.1% | 2030 Value Projection: | US$ 2,293.56 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Novartis International AG, Merck & Co., Inc., Aimmune Therapeutics, Inc., Regeneron Pharmaceuticals, Inc., HAL Allergy B.V., Stallergenes Greer, ALK-Abelló A/S, Allergy Therapeutics Plc, Torii Pharmaceutical Co., Ltd., and DESENTUM OY |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Figure 1: Europe and Japan Allergy Immunotherapy Market Share, (%), Analysis, By Treatment Type, 2022
To learn more about this report, Download Free Sample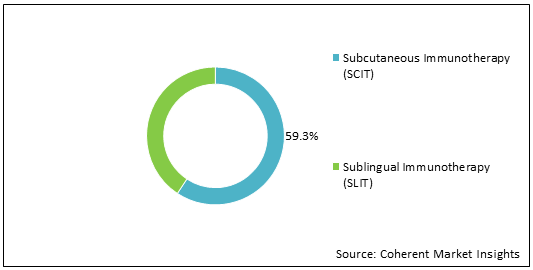
Market players are conducting clinical trials for development of treatment for allergy immunotherapy. This is expected to drive growth of the Europe and Japan allergy immunotherapy market over the forecast period.
Market players are conducting clinical trials for development of immunotherapy treatment for various allergies like allergic rhinitis, pet allergies, food allergies etc. for instance, the following table shows some of the clinical trials that are being conducted by the market players for allergy immunotherapy.
| Title | Number of Cases | Sponsors | Phases | Completion Date | ||
| A Study of the Safety and Feasibility of Up-titration With INT301 in Adults With Sensitivity to Peanut | Peanut Allergy | Intrommune Therapeutics | Phase I |
August 15, 2022 |
||
| ADP101 for Oral Immunotherapy in Food-Allergic Children and Adults | Food Allergy | Alladapt Immunotherapeutics, Inc. | Phase I / II |
December 12, 2022 |
||
| Peanut Oral Immunotherapy Study of Early Intervention for Desensitization | Peanut Allergy | Aimmune Therapeutics, Inc. | Phase III |
June 22, 2022 |
||
| Oral Immunotherapy for Peanut Allergic Patients | Peanut Allergy | InnoUp Farma S.L. | Phase I / II |
June 22, 2022 |
||
| Open-label Extension Study of ADP101 | Food Allergy | Alladapt Immunotherapeutics, Inc. | Phase I / II |
December 26, 2022 |
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Key players are engaged in receiving approvals for their products from regulatory authorities and this is expected to drive growth of the Europe and Japan allergy immunotherapy market over the forecast period.
Increasing number of marketing authorizations by regulatory authorities, is expected to increase growth of market, over the forecast period. For instance, in 2018, HAL Allergy B.V. received the registration of SUBLIVAC Birch 40.000 AUN/ml and SUBLIVAC Trees 40.000 AUN/ml in Germany. The marketing authorizations were granted by German Therapieallergene Verordnung (TAV), which is the German Federal Ministry of Health, which regulates the marketing authorization requirements for the frequent therapeutic allergens.
Europe and Japan Allergy Immunotherapy Market – Restraints
Side effects associated with allergy immunotherapy drugs is expected to hamper growth of the Europe and Japan allergy immunotherapy market, over the forecast period. Side effects associated with sublingual immunotherapy (SLIT) are expected to restrain growth of the Europe and Japan allergy immunotherapy market over the forecast period. The most common adverse effects of sublingual immunotherapy (SLIT) are oral pruritus, ear pruritus, throat irritation, and swelling of the lips, tongue, and pharynx. A smaller percentage of patients may also experience edema of the tongue, uvula, lips, or throat that may occasionally require epinephrine. Furthermore, according to an article published by National Center for Biotechnology Information: 2019, in the U.S., it was reported that 4 to 7% of patients dropped out of the clinical trial due to the adverse effects of immunotherapy for allergies
Europe and Japan Allergy Immunotherapy Market – Regional Analysis
Among regions, Europe allergy immunotherapy market is expected to hold a dominant position during the forecast period, owing to increasing marketing authorization by the regulatory authorities. For instance, in June 2019, ALK-Abelló A/S announced that it successfully completed the marketing authorization procedure for its tree sublingual allergy immunotherapy (SLIT) tablet in 17 European countries. ITULAZAX is expected to be the brand name of the tree SLIT-tablet.
Figure 2: Europe and Japan Allergy Immunotherapy Market (US$ Mn), by Region, 2022
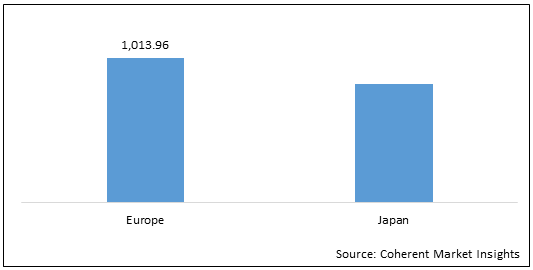
To learn more about this report, Download Free Sample
Europe and Japan Allergy Immunotherapy Market – Competitive Landscape
Major players operating in the Europe and Japan allergy immunotherapy market include Novartis International AG, Merck & Co., Inc., Aimmune Therapeutics, Inc., Regeneron Pharmaceuticals, Inc., HAL Allergy B.V., Stallergenes Greer, ALK-Abelló A/S, Allergy Therapeutics Plc, Torii Pharmaceutical Co., Ltd., and DESENTUM OY.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients