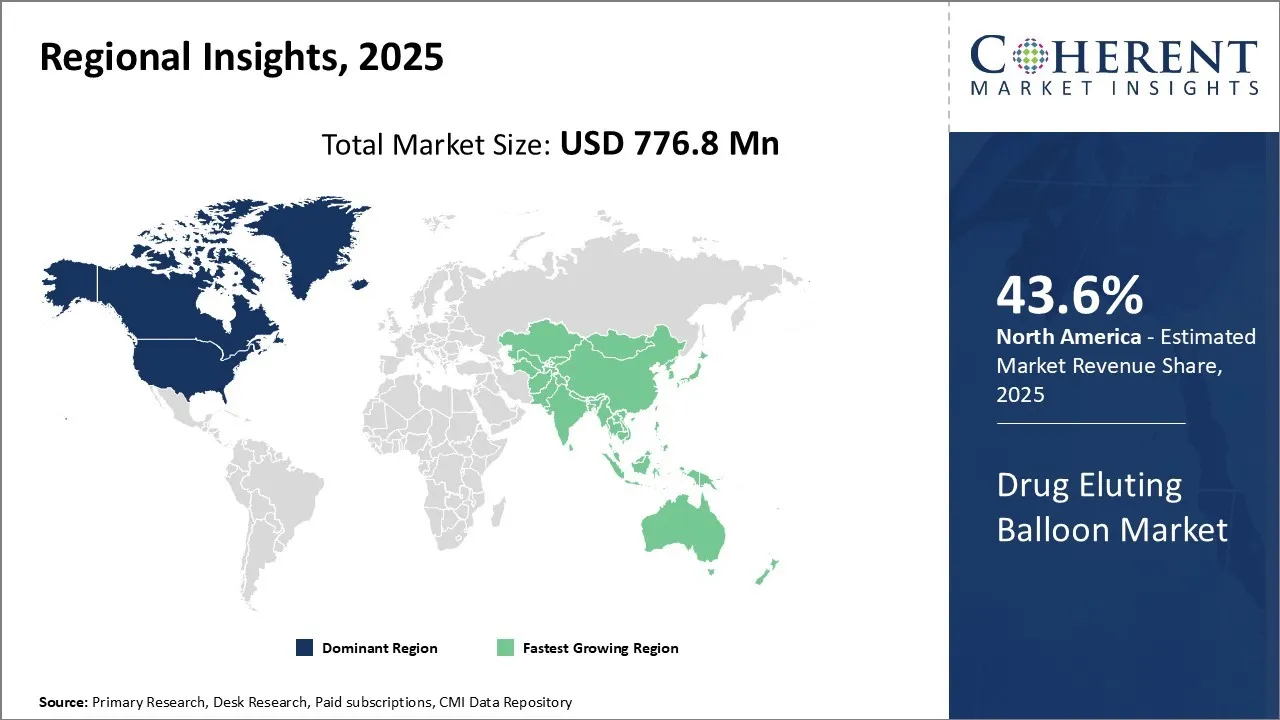
Global drug eluting balloon market is estimated to be valued at USD 776.8 Mn in 2025 and is expected to reach USD 1,402.8 Mn by 2032, exhibiting a compound annual growth rate (CAGR) of 8.8% from 2025 to 2032.
An increase in the use of drug eluting balloons can be seen because there are currently more cases of cardiovascular diseases (CVDs) globally. It is estimated by global health authorities that CVDs lead to the deaths of more than 17.9 million people every year which makes them the leading cause of death. Older people, inactive lifestyles, unhealthy eating and more obesity have greatly increased the rate of both coronary artery and peripheral artery diseases. Drug eluting balloons (DEBs) have been favored in this situation because they are minimally invasive and provide treatment without leaving behind anything. Since stents aren’t used in this way, patients in higher-risk and elderly groups have fewer long-term problems and recover more easily.
|
Current Events |
Description and its impact |
|
Geopolitical Tensions and Supply Chain Disruptions |
|
|
Economic Pressures and Cost-containment Measures by Healthcare Providers |
|
|
Technological Advances Impacting Drug Coating and Delivery |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Clinicians and hospital procurement teams have highlighted several unmet needs in the current DEB landscape. According to interviews with interventional cardiologists and data from multi-center trials like PACT SFA II and BIOLUX P-III, users report high satisfaction with DEB outcomes in ISR and small vessel disease, citing fewer repeat procedures and lower late lumen loss. However, they express concern about limited DEB options for larger vessels or calcified lesions, as well as limited product availability in Tier 2 and Tier 3 cities. Furthermore, nurses and surgical staff in ambulatory surgical centers note the need for DEBs with better crossing profiles and lower preparation times. The push for same-day discharge has elevated demand for more efficient and ergonomically packaged DEB kits.
Product Type segment is sub-segmented into coronary drug eluting balloon, peripheral drug eluting balloon, others. Coronary drug eluting balloon sub-segment is estimated to hold 47.8% of the market share in 2025, owing to the growing need for minimally invasive treatments. Drug eluting balloons address this challenge by utilizing drug coated non-expandable balloons to treat lesions in a single procedure. Upon inflation, the drug is infused to the vessel walls to prevent narrowing of arteries without leaving behind a metallic implant. This saves patients from undergoing multiple procedures and allows for faster recovery times. The one-time therapy increases treatment adherence and compliance for chronic PAD patients who are often elderly with comorbidities.
The U.S. FDA's approval of Boston Scientific's Agent™ drug-coated balloon catheter for treating coronary in-stent restenosis (ISR) underscores the growing clinical adoption of DEBs in managing complex coronary sessions, further enhancing the drug eluting balloon market share.
Technology segment is sub-segmented into freepac, transpac, enduracoa, others. Freepac sub-segment is estimated to hold 39.8% of the market share in 2025 due to its proven superior clinical outcomes. Among drug coating technologies, FreePac uses an innovative approach without a guiding catheter to infuse drugs onto the balloon surface. Unlike competitive TransPac or EnduraCoat technologies which rely on enclosed drug reservoirs, FreePac allows for homogenous drug transfer unconstrained by physical borders or flow restrictions. Several randomized control trials have demonstrated FreePac's ability to achieve high vessel wall concentrations with extensive, transmural drug transfer and coverage. Its unrestricted fluid dynamics minimizes residual ducts and dead spaces, addressing shortcomings of traditional systems. As a result, FreePac has shown advantages in reducing risk of target lesion revascularization compared to competing technologies. It offers durable clinical results with low vascular retreatment needs over extended follow up periods. Medtronic's recent expansion of CE Mark indications for its Prevail™ paclitaxel-coated PTCA balloon catheter underscores the growing traction of FreePac™ technology in the drug-eluting balloon (DEB) market. The Prevail™ catheter is now approved for treating complex bifurcation lesions, a challenging subset of coronary artery disease, highlighting the device's efficacy in intricate vascular anatomies. This is further propelling the drug eluting balloon market demand.
End User segment is sub-segmented into hospitals, ambulatory surgical centers, others. Hospitals sub-segment is estimated to hold 36.2% of the market share in 2025 as these are the preferred settings for drug eluting balloon procedures. While ambulatory surgical centers also provide such services, hospitals offer logistic and personnel infrastructure required for complex cases, especially in high-risk patients. Their large scale allows for economies needed to support technological innovations like drug eluting balloons. Importantly, hospitals enable treatment in outpatient settings which is driving increased adoption. Drug eluting balloons enable same day discharge for many patients due to speed and minimally invasive nature versus stent or surgery alternatives. This reduces burden on inpatient beds and wait times. Favorable reimbursements for such same day discharges further incentivize hospitals to incorporate such technologies. The use of preoperative DEB therapy in patients undergoing elective tumor surgery with concurrent coronary heart disease. The research demonstrated that hospitals equipped with the necessary infrastructure could effectively administer DEB treatments, leading to favorable perioperative outcomes and reduced cardiac complications. This is further escalating the drug eluting balloon industry growth.
To learn more about this report, Download Free Sample
North America remains the dominant region in the global drug eluting balloon market and is estimated to hold 43.6% of the market share in 2025 due to high healthcare expenditure, presence of leading players and adoption of advanced technologies at faster pace in countries like U.S. and Canada. Rising prevalence of cardiovascular diseases in North America contributes to larger patient pool undergoing angioplasty procedures, where drug eluting balloons are widely used. North America is an attractive base for many international players to set up manufacturing facilities due to availability of key resources and skilled workforce. The launch of Cordis' 10,000-patient global coronary registry for the SELUTION SLR™ drug-eluting balloon (DEB) underscores North America's pivotal role in advancing DEB technologies. This extensive registry aims to collect real-world data on the safety and efficacy of the SELUTION SLR™ DEB, reflecting the region's commitment to evidence-based medical advancements.
One of the fastest growing regions for drug eluting balloon market is Asia Pacific. The growth can be attributed to rising healthcare infrastructure, growing medical tourism industry and increasing disposable incomes in developing economies like China and India. Both China and India have a huge population base with growing disease burdens and rising cases of lifestyle diseases. This serves as a huge patient pool and potential market for all medical devices including drug eluting balloons. Additionally, many Asian countries are focusing on enhancing healthcare access through various governmental initiatives, and this boosts demand for cost-effective treatment alternatives like drug eluting balloons. Reflow Medical's establishment of its European subsidiary in Landsberg am Lech, Germany, marks a strategic expansion that extends beyond Europe, targeting the Asia-Pacific (APAC) region among others. This move is poised to influence the APAC drug-eluting balloon (DEB) market by enhancing access to Reflow's innovative cardiovascular devices, including the Spur® Peripheral Retrievable Scaffold System.
The United States represents one of the most advanced markets for drug eluting balloon (DEB) technologies, driven by a combination of robust healthcare infrastructure, high disease prevalence, and early adoption of minimally invasive interventions. The increasing incidence of coronary artery disease (CAD) and peripheral artery disease (PAD), particularly among aging populations and those with comorbidities such as diabetes and obesity, has spurred demand for alternative treatment options that avoid permanent implants. Drug eluting balloons offer a compelling solution by enabling localized drug delivery without leaving a stent behind, thereby reducing risks associated with stent-related complications such as restenosis and thrombosis. The completion of U.S. enrollment in the PACT SFA II trial marks a significant milestone in the advancement of drug-eluting balloon (DEB) therapies within the United States. This prospective, multi-center, randomized controlled trial enrolled 181 patients across more than 40 sites, evaluating the efficacy of paclitaxel-coated balloons in treating superficial femoral artery (SFA) lesions. The successful enrollment underscores the growing clinical interest and adoption of DEB technologies in U.S. vascular interventions.
India’s drug eluting balloon market is rapidly evolving, supported by a growing healthcare infrastructure, increasing prevalence of cardiovascular and peripheral vascular diseases, and the nation’s strong push toward affordable and minimally invasive surgery options. With a large and aging population, and rising incidence of lifestyle-related conditions such as diabetes and hypertension, the country is witnessing greater demand for advanced interventional cardiology solutions. DEBs are gaining traction as a stent-free alternative that minimizes hospital stays and reduces long-term follow-up care, making them particularly appealing in resource-constrained settings. SIRONA trial signifies a pivotal advancement in India's drug-eluting balloon (DEB) market, particularly in the treatment of peripheral artery disease (PAD). The trial's findings position sirolimus-coated balloons (SCBs) as a viable alternative to the traditionally used paclitaxel-coated balloons, addressing previous safety concerns associated with paclitaxel.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 776.8 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.8% | 2032 Value Projection: | USD 1,402.8 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Boston Scientific Corporation, BD, Terumo Corporation, Medtronic, B. Braun SE, Surmodics, Inc., Biotronik, Cook Medical, Opto Circuits (India) Limited, Lepu Medical Technology, Cardionovum GmbH, Shanghai MicroPort Medical (Group) Co., Ltd., Wellinq, Philips Healthcare, Biosensors International Group, Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Increasing prevalence of cardiovascular diseases across the globe is expected to drive the growth of the drug eluting balloon market. World Health Organization, cardiovascular diseases account for over 17.9 million deaths each year globally. Some of the major cardiovascular conditions include coronary heart disease, stroke and peripheral arterial disease. Aging populations, changing environmental factors and genetic predispositions further contribute to rising disease burden. Drug eluting balloons are being increasingly used by physicians for treating peripheral arterial diseases affecting the hips and legs as well as coronary artery diseases impacting the heart. These prevent vessel recoil and neointimal hyperplasia after angioplasty procedures through the controlled, localized delivery of anti-proliferative drugs to damaged vessel sites. This minimizes the risk of restenosis and the need for additional revascularization treatments. Recent clinical studies have demonstrated drug eluting balloons to be as effective as drug eluting stents in addressing various types of atherosclerotic lesions with fewer safety issues.
The Drug Eluting Balloon Market forecast reflects a strong upward trajectory, fueled by aggressive inorganic growth strategies among leading players. Companies are pursuing mergers, acquisitions, strategic partnerships, and licensing deals to expand their product portfolios and global reach. For instance:
These inorganic moves are reshaping competitive dynamics and accelerating innovation, positioning the market for sustained growth and broader clinical adoption.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients