Anti-Radiation Drugs Market is estimated to be valued at USD 669.5 Mn in 2025 and is expected to reach USD 1,061.1 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 6.8% from 2025 to 2032.
Anti-radiation drugs are used to treat the effects of radiation exposure from nuclear accidents, radiation therapy, or nuclear warfare. These protect healthy cells and tissues from the damaging effects of radiation exposure. The key drivers of this market include rising incidence of cancer, increasing number of nuclear accidents, robust drug pipeline, and large target patient pool.
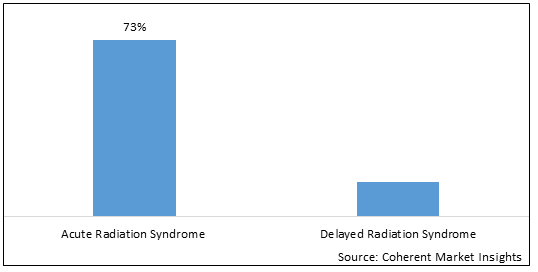
Global anti-radiation drugs market is segmented into indication, route of administration, distribution channel, and region. By indication, the market is segmented into acute radiation syndrome and delayed radiation syndrome. The acute radiation syndrome segment accounted for the largest market share in 2025. Acute Radiation Syndrome (ARS) (sometimes known as radiation toxicity or radiation sickness) is an acute illness caused by irradiation of the entire body (or most of the body) by a high dose of penetrating radiation in a very short period of time (usually a matter of minutes).
Global Anti-Radiation Drugs Market- Regional Insights
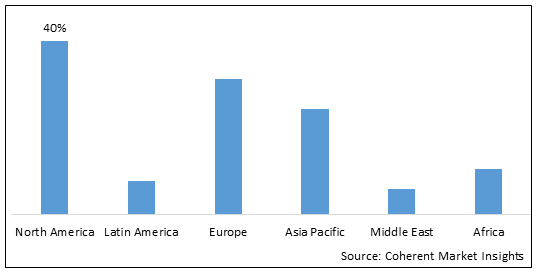
- North America is expected to be the largest market for anti-radiation drugs market during the forecast period, and accounted for over 40% of the market share in 2025. North America market growth is attributed to high adoption of radiation therapy, increasing prevalence of cancer, and presence of leading market players.
- The Europe market is expected to be the second-largest market for anti-radiation drugs market, and accounted for over 30% of the market share in 2025. Europe market growth is attributed to rising cases of cancer, supportive government initiatives, and investments in R&D.
- The Asia Pacific market is expected to be the fastest-growing market for anti-radiation drugs market, with a CAGR of over 15% during the forecast period. Asia Pacific market growth is attributed to improving healthcare infrastructure, rising disposable incomes, and increasing awareness about radiation therapy.
Analyst View’s
Anti-radiation drugs are used to treat radiation syndrome, and currently there is a lack of treatment. Due to this, investors are focusing on developing new and more effective anti-radiation drugs by bringing new clinical trials to the market. These clinical trials aim to test the safety and efficacy of potential anti-radiation drugs with the hope of finding a breakthrough treatment that can mitigate the harmful effects of radiation exposure. The development of new and more effective anti-radiation drugs is crucial in order to provide better medical options for individuals affected by radiation syndrome and improve their chances of recovery. In addition, the development of these drugs is essential for addressing the growing concerns surrounding nuclear accidents and radiation-related incidents. By investing in clinical trials and research, we can enhance our understanding of radiation's impact on the human body and develop targeted treatments that can minimize long-term health consequences. Ultimately, these advancements will not only benefit individuals affected by radiation syndrome but also contribute to global efforts in preparedness and response to potential radiological emergencies.
Figure 1. Global Anti-Radiation Drugs Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
Global Anti-Radiation Drugs Market- Drivers
- Increasing incidence of cancer globally: Rising prevalence of cancer across the globe is a major factor that boosts demand for anti-radiation drugs. Radiation therapy is commonly used to treat cancer, either alone or in combination with other treatments like chemotherapy or surgery. Increasing use of radiation therapy for cancer treatment is expected to boost adoption of radioprotectants and mitigators to manage the side effects of radiation exposure in normal tissues. For instance, according to the article published by European Cancer Information System, in 2022, new cancer cases increased by 2.3 % as compared to 2020, to reach 2.74 million in 2022. Similarly, cancer deaths increased by 2.4 % as compared to 2020.
- Growing threat of nuclear accidents and events: The potential threat of nuclear accidents and warfare increases need for drugs to treat acute radiation syndrome. Though the use of nuclear weapons has declined since the cold war, the risk of nuclear terrorism and accidents at nuclear plants has increased. The Fukushima nuclear disaster in 2011 increased the focus on being prepared for nuclear emergencies. Government agencies are stockpiling anti-radiation drugs as a part of medical countermeasures. This is expected to propel the anti-radiation drugs industry.
- Favorable regulatory environment for drug development: Regulatory bodies like the U.S. FDA and EMA are encouraging the development of novel countermeasures against radiation exposure. Designations like fast track, breakthrough therapy, and orphan drug status help to expedite regulatory review. Companies are receiving grants from government bodies like Biomedical Advanced Research and Development Authority (BARDA) and the National Institutes of Health to advance R&D. Such initiatives are expected to aid the growth of the anti-radiation drugs pipeline.
- Technological advancements in drug delivery systems: Advances in nanoparticle technology, liposomal encapsulation, polymeric micelles, and microfluidic systems leads to emergence of sophisticated drug delivery techniques for anti-radiation molecules. Platforms like ThermoVax allow targeted delivery to increase drug concentration in organs susceptible to ARS. Advanced delivery systems can enhance the effectiveness and safety profile of anti-radiation compounds. This is expected to widen the clinical applications of these drugs.
Global Anti-Radiation Drugs Market- Opportunities
- Development of effective countermeasures for emerging radiological threats: The use of radioactive compounds in dirty bombs by terrorist organizations represents an emerging threat. Radioactive materials like Cesium-137 and Cobalt-60 can cause radiation sickness and environmental contamination. There is an unmet need for safe and efficacious drugs to protect civilians and first responders from dirty bomb detonations. Pharmaceutical companies have an opportunity to develop specific antidotes and treatments to prepare for such radiological emergencies.
- Rising demand for supportive care during radiation therapy: Only a few drugs like amifostine are approved to manage the side effects of radiation therapy in cancer patients. There is considerable opportunity for biopharma companies to develop novel compounds that can minimize the damage to normal tissue during radiation treatment, improving quality of life and enabling higher radiation doses for better tumor control.
- Combination therapies for better clinical outcomes: Combining radioprotective and radiosensitizing drugs is an emerging opportunity to enhance the safety and efficacy of radiation therapy in oncology. For instance, combining Amifostine with radiosensitizing platinum chemotherapy may allow higher tumor regression while protecting healthy tissues. Development of such synergistic combination therapies can significantly expand the clinical utility of anti-radiation drugs.
- Expanding applications in space travel, aviation and defense: Exposure to cosmic ionizing radiation during space missions increases the risk of ARS, cancer and organ damage in astronauts. Anti-radiation drugs can potentially allow longer duration human space travel. These drugs also have applications in aviation, military and civil defense to protect personnel against the effects of a nuclear event. Antiradiation drugs have an extra growth route due to the specific needs of these markets.
Anti-Radiation Drugs Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 669.5 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.8% | 2032 Value Projection: | USD 1,061.1 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Amgen Inc., Partner Therapeutics Inc., RedHill Biopharma Ltd., Humanetics Corporation, RxBio Inc., Soligenix Inc., Aeolus Pharmaceuticals, Onconova Therapeutics, Genome Protection Inc., and others. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Anti-Radiation Drugs Market- Trends
- Accelerated research on radiomitigators showing delayed efficacy: Most investigational anti-radiation agents demonstrated efficacy when administered before exposure. However, in the event of a surprise nuclear detonation or accident, pretreatment is not possible. There is a growing focus on radiomitigators that can be provided after exposure and still provide meaningful protection by mitigating delayed effects. Research is focused on drugs that can suppress prolonged inflammation and oxidative stress.
- Shift towards natural and plant-based radio-protectants: Synthetic radioprotective compounds often have undesirable side effects. Hence, there is increasing research on plant-derived molecules like flavonoids, antioxidants and polysaccharides that can enhance the resistance of normal tissues to radiation. Compounds derived from ginseng, orientin, bromelain and many other botanicals are being evaluated as natural radioprotective agents. The goal is to develop effective and safe agents leveraging traditional medicine. For instance, Journal of pharmacy & bioallied sciences 2021, showed that natural herbal products are nontoxic with proven therapeutic benefits and have long been used to treat various diseases.
- Emergence of system biology and OMICS approaches: Conventional pharmacological research is being combined with systems biology and OMICS techniques like transcriptomics, proteomics and metabolomics to identify novel molecular targets and biomarkers for anti-radiation drug discovery. Such interdisciplinary approaches are providing unprecedented insight into mechanisms of radiation injury and action of radioprotective agents in humans. This can support efficient development of effective countermeasures.
- Selective protection of radiosensitive organs: Rather than systemic protection, there is rising focus on developing drugs that can selectively shield organs most susceptible to acute and delayed effects of radiation such as bone marrow, gastrointestinal tract, lungs, skin and eyes. For instance, oral mucosal radioprotectant gel is being developed to prevent mucositis during head & neck radiation therapy. Such targeted organ protection can improve patient outcomes.
Global Anti-Radiation Drugs Market- Restraints
- Challenges in clinical evaluation of anti-radiation drugs: Conducting controlled clinical trials for therapies against acute radiation syndrome poses ethical and practical challenges. Radiation exposure in human subjects without therapeutic necessity raises ethical concerns. The sporadic nature of nuclear accidents makes data collection difficult. Approval is often based on efficacy in animal models, which do not fully represent human radiation response. Lack of human clinical data can restrain adoption in medical practice.
- Toxicities and adverse effects associated with anti-radiation compounds: Most synthetic radiation protecting and mitigating agents have side effects like nausea, vomiting, hypotension and neurotoxicity that can limit their use. For example, Amifostine can cause nausea and vomiting in more than 50% patients. Even natural compounds like those derived from green tea and berry extracts can have interactions with chemotherapy drugs. Concerns regarding potential toxicity and adverse effects can limit clinical potential of these agents.
- Limited mechanistic understanding of radiation injury pathogenesis: The exact mechanisms behind the pathophysiology of acute radiation syndrome and delayed radiation injuries are still unclear. Knowledge gaps exist with respect to molecular pathways of normal tissue radiation damage, biomarker expression and pharmacological targets. Deficiencies in understanding radiation sickness pathology poses challenges during drug discovery efforts for medical countermeasures targeting ARS.
Market key players are now focusing on delivering effective, and less toxic radiation associated drugs to bring the patient life to normal stage. And, also investors are continuously investing funds to understand the MoA.
Global Anti-Radiation Drugs Market- Recent Developments
Clinical Developments
- On September 06, 2023, Humanetics Corporation, a clinical-stage specialty pharmaceutical company, announced that the company was awarded with a 5-year contract from the Department of Defense (DOD) to develop its drug, BIO 300, as a medical countermeasure to prevent bodily harm caused by acute exposure to radiation. The contract is in the form of an Other Transaction Authority (OTA) for Prototype Agreement. It includes a base period of US$ 20 million for activities required to gain U.S. Food and Drug Administration (FDA) Emergency Use Authorization for the use of BIO 300 under a potential military emergency. The OTA agreement provides options for the DOD to fund all activities required to bring BIO 300 to full FDA approval.
- On February 15, 2023, RedHill Biopharma Ltd., a specialty biopharmaceutical company, announced the positive outcome of a scheduled Type B meeting with the U.S. Food and Drug Administration (FDA) for the development of opaganib for Acute Radiation Syndrome (ARS) in which the FDA provided guidance on opaganib's developmental pathway to potential approval under the Animal Rule
- In March 2022, Tanner Pharma Group, an international distributor of essential medicines, announced that it had significantly increased its inventory of Leukine (sargramostim, yeast-derived rhuGM-CSF) to be held in Europe. This action is being taken in partnership with Leukine’s owner, Partner Therapeutics (PTx), in response to the ongoing war in Ukraine and escalating potential for incidents that could require rapid deployment of medical interventions to treat radiation or chemical exposure.
- In February 2022, Partner Therapeutics, Inc. (PTx), a commercial biotech company, announced the publication of two pivotal studies that provided the basis for approval of Leukine (sargramostim, yeast-derived rhu GM-CSF) to improve survival in patients exposed to myelosuppressive doses of radiation (Hematopoietic syndrome of acute radiation syndrome [H-ARS]). The first study, “Sargramostim (rhu GM-CSF) Improves Survival of Non-Human Primates with Severe Bone Marrow Suppression after Acute, High-Dose, Whole-Body Irradiation” (Clayton, et al.) was published in Radiation Research. The second study, “Efficacy of Delayed Administration of sargramostim up to 120 hours Post Exposure in a Nonhuman Primate Total Body Radiation Model” (Zhong, et al.), was published in International Journal of Radiation Biology.
Acquisition, Partnerships, and Funding
- On September 12, 2023, University of Nebraska–Lincoln, National Strategic Research Institute and the University of Nebraska Medical Center, had received a US$ 24.5 million award from the Defense Health Agency to advance development of an acute radiation syndrome prophylactic. The University of Nebraska research team will develop a first-of-its-kind treatment aimed at protecting U.S. troops from the effects of acute radiation syndrome.
- On July 11, 2023, Pluri, a Israeli company engaged in the development of human placental adherent stromal cells for commercial use in disease treatment, signed a three-year US$ 4.2m contract with the U.S. National Institute of Allergy and Infectious Diseases (NIAID) and the US Department of Defense (DoD) to develop its cell therapy avoplacel (PLX-R18) to treat haematopoietic acute radiation syndrome or radiation poisoning.
- In November 2022, Researchers with The Ohio State University Comprehensive Cancer Center –Arthur G. James Cancer Hospital and Richard J. Solove Research Institute (OSUCCC – James) had entered a federal cooperative agreement, valued up to US$ 9.42 million, that will help to further develop Ohio State’s biodosimetry technology to discover noninvasive biomarkers for radiation exposure – work that will have national security applications
Figure 2. Global Anti-Radiation Drugs Market Share (%), By Indication, 2025
To learn more about this report, Download Free Sample
Top companies in Global Anti-Radiation Drugs Market
- Amgen Inc.
- Partner Therapeutics Inc.
- RedHill Biopharma Ltd.
- Humanetics Corporation
- RxBio Inc.
- Soligenix Inc.
- Aeolus Pharmaceuticals
- Onconova Therapeutics
- Genome Protection Inc.
Definition: Anti-radiation drugs refers to the drugs used to treat the damaging effects of radiation exposure. Radiation exposure can occur during radiation therapy for cancer, nuclear accidents, or nuclear warfare. Anti-radiation drugs help TO protect healthy tissues from ionizing radiation damage by reducing oxidative stress, inflammation, and cell death.
Few other promising reports in Pharmaceutical Industry
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients




