Blood Collection Devices Market Size and Forecast – 2026 – 2033
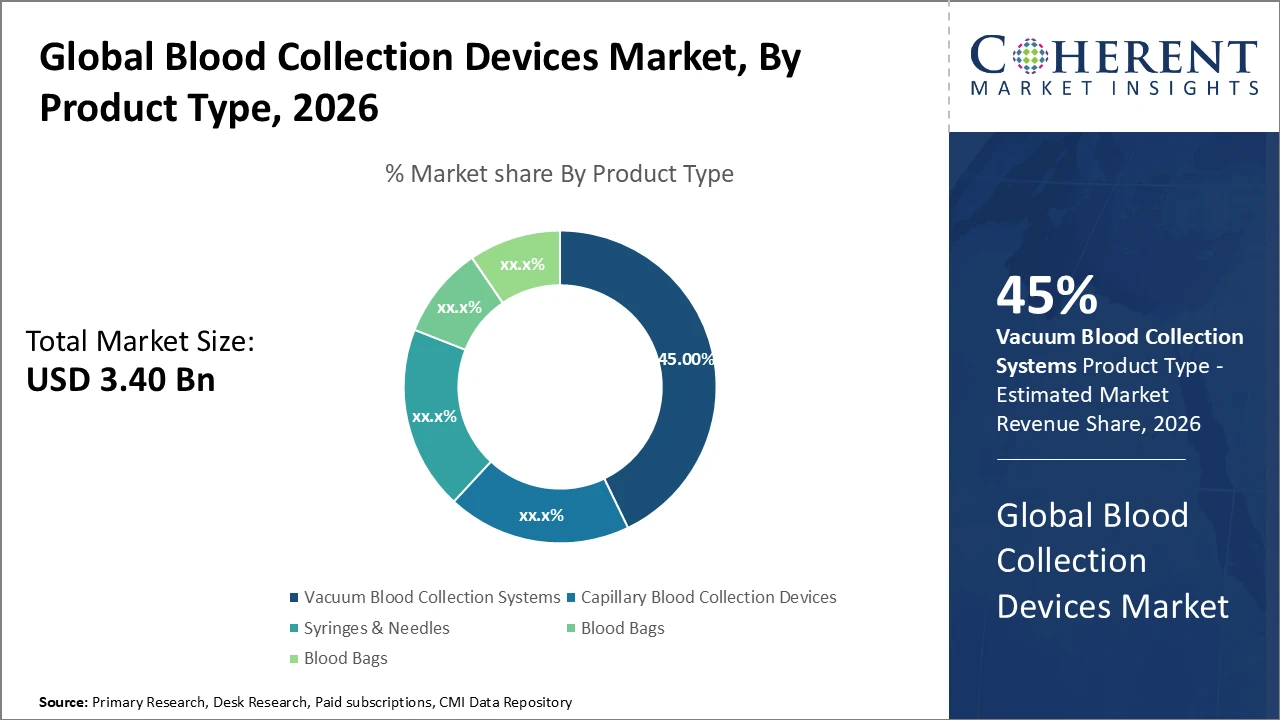
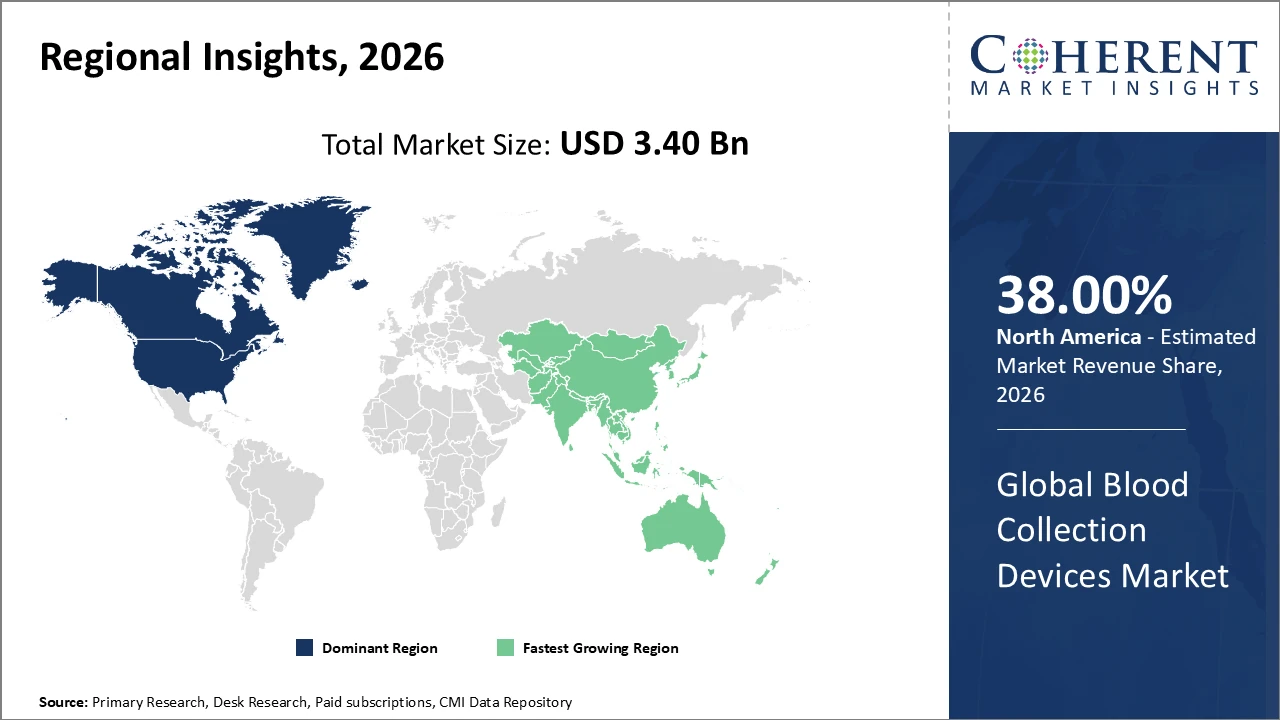
The global blood collection devices market is estimated to be valued at USD 3.40 billion in 2026 and is expected to reach USD 5.70 billion by 2033, exhibiting a compound annual growth rate (CAGR) of 7.4% from 2026 to 2033.
Global Blood Collection Devices Market Overview
The blood collection devices market is witnessing steady growth, driven by increasing demand across hospitals, diagnostic laboratories, and in-home healthcare. The market is propelled by advancements in technology, a growing focus on patient safety, and the need for efficient and reliable blood collection methods. Key trends include the integration of automation to streamline workflows and the adoption of biocompatible and environmentally friendly materials to minimize contamination risks and enhance user safety. Rising awareness of proper blood collection practices further supports market expansion.
Key Takeaways
Vacuum blood collection systems dominate the product segment, holding around 45% of the market due to their efficiency and strong safety profile.
Hospitals are the largest end users, representing about half of the market volume because of centralized testing and standardized procedures.
North America generates the highest regional revenue, contributing close to 38% of the market, supported by advanced healthcare infrastructure and regulatory frameworks.
Asia Pacific is the fastest-growing region, with growth driven by rising healthcare investments, expanding diagnostic facilities, and favourable government policies.
Blood Collection Devices Market Segmentation Analysis
To learn more about this report, Download Free Sample
Blood Collection Devices Market Insights, By Product Type
Vacuum blood collection systems dominate the market, accounting for 45% of the share due to their reliability, ease of use, and enhanced patient safety. These systems are favored for their seamless workflow integration in high-volume testing facilities and improved sample integrity. Capillary blood collection devices are the fastest-growing subsegment, driven by increasing demand in pediatric and geriatric testing, as well as home-based and point-of-care diagnostics. Syringes and needles remain essential for specialized procedures requiring precision, while blood bags are primarily used for transfusions in hospital settings.
Blood Collection Devices Market Insights, By Material Type
Plastic materials hold the largest market share due to their cost-effectiveness, disposability, and compliance with safety standards. Their versatility and compatibility with automated systems drive widespread adoption across various segments. Glass remains important for high-precision and long-term storage applications but is limited by its breakage risk.
Blood Collection Devices Market Insights, By End-User
Hospitals account for 50% of the market, driven by centralized processing protocols, strict safety compliance, and high-volume sample collection demands. Diagnostic laboratories are steadily expanding, supported by increased outsourcing of testing services and adoption of automated collection workflows. Ambulatory surgical centers form a niche but growing segment due to the rise in outpatient procedures requiring preoperative blood testing. The home healthcare sector is the fastest-growing subsegment, fueled by the expansion of telemedicine services and patient preference for convenient, minimally invasive collection methods.
Blood Collection Devices Market Trends
Automation technologies are a key market trend, with hospitals using automated blood collection systems reporting up to 20% faster sample processing, improving patient throughput.
The rise of telehealth services has driven increased adoption of at-home blood collection devices, which grew by nearly 25% over the past two years.
Eco-friendly initiatives are promoting the use of biodegradable and recyclable materials in consumables, supporting global sustainability regulations.
These trends are shaping market dynamics by encouraging product innovation and expanding applications across healthcare settings.
Blood Collection Devices Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Blood Collection Devices Market Analysis and Trends
North America leads the blood collection devices market, driven by established healthcare infrastructure, robust regulatory frameworks, and substantial R&D investments by domestic companies. The region holds approximately 38% of the overall market share, supported by widespread adoption of automated systems and safety-engineered devices. Strong reimbursement policies and proactive government health initiatives further reinforce North America’s leadership position in the market.
Asia Pacific Blood Collection Devices Market Analysis and Trends
The Asia Pacific region is the fastest-growing market for blood collection devices, driven by expanding hospital networks, increased government investment in healthcare infrastructure, and a rising prevalence of chronic diseases. India and China are the leading contributors, fuelled by growing diagnostic testing demand and cost-effective local manufacturing. Supportive trade policies and the entry of international market players have further strengthened the region’s position, making Asia Pacific a key hub for industry expansion and innovation.
Blood Collection Devices Market Outlook for Key Countries
USA Blood Collection Devices Market Analysis and Trends
The U.S. blood collection devices market is driven by advanced clinical research infrastructure and stringent patient safety requirements, which have accelerated the adoption of safety-engineered systems. FDA guidelines introduced in 2025, emphasizing device sterility and efficient waste management, have prompted manufacturers to develop innovative materials and sustainable solutions. Leading U.S.-based companies hold a substantial share of market revenue, while healthcare providers increasingly leverage automation to streamline diagnostic workflows. The launch of next-generation devices with multi-sample capabilities further contributes to market expansion and revenue growth.
Germany Blood Collection Devices Market Analysis and Trends
Germany’s blood collection devices market is experiencing strong growth, driven by its leadership in precision healthcare and well-established hospital networks. Government incentives for early diagnostic technologies and the expansion of outpatient diagnostic centers have increased demand for vacuum and safety blood collection devices. Local manufacturers are investing in advanced polymer technologies, producing environmentally sustainable devices that comply with strict EU regulations introduced in 2024. Collaboration between research institutions and industry players has further accelerated new product development, strengthening Germany’s market share domestically and across Europe.
Analyst Opinion
Efficient Supply Chain Dynamics: Expansion of production capacity, particularly in Asia Pacific manufacturing hubs, has increased output to meet growing global demand. Strategic pricing balances raw material cost fluctuations with competitive product pricing, while export volumes from leading manufacturing countries rose, further strengthening market share.
Diverse Clinical and Diagnostic Use Cases: Blood collection devices are increasingly utilized in ambulatory care, emergency services, and chronic disease monitoring. Point-of-care testing devices have seen notable growth, driven by rising demand from diabetic and oncology patient segments.
Technological Advancements Driving Adoption: Safety-engineered needles and vacuum-assisted systems have contributed to a significant reduction in needlestick injuries, enhancing compliance with occupational safety regulations and encouraging broader adoption in healthcare facilities.
Regulatory Influence and Market Refinement: Stricter standards on biocompatibility and device sterility across Europe and the U.S. have accelerated product innovation. Manufacturers are incorporating advanced polymer composites, reflected in a marked increase in patent filings related to blood collection technologies.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 3.4 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 7.4% | 2033 Value Projection: | USD 5.7 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Becton, Dickinson and Company, Terumo Corporation, Abbott Laboratories, Cardinal Health, Inc., Nipro Corporation, 3M Company, Siemens Healthineers, Bio-Rad Labs, BioIVT, Cenova Diagnostics, Fujifilm Corporation | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Blood Collection Devices Market Growth Factors
The rising prevalence of chronic diseases, including diabetes and cardiovascular conditions, is driving increased adoption of blood collection devices, with diabetes-related testing showing significant growth. Growing demand for minimally invasive diagnostics and patient-centric care is boosting the use of capillary blood collection devices. Additionally, government initiatives supporting early disease detection and enhanced laboratory networks, particularly in emerging markets, are creating new growth opportunities. Advances in technology that improve safety and ease of use are encouraging healthcare institutions to adopt updated blood collection systems, reflected in the steady rise of safety-engineered needle utilization.
Blood Collection Devices Market Development
In January 2025, Vitestro unveiled its new website and released the first public video of Aletta, an autonomous robotic phlebotomy device. Designed to automate blood collection, Aletta enhances efficiency, reduces human error, and improves patient experience. This innovation marks a significant advancement in medical robotics and diagnostic sample collection technology.
In July 2025, OraSure Technologies launched a novel blood collection device designed for proteomic applications. The innovation enhances sample quality, enabling reliable protein analysis for biomarker discovery, drug development, and precision medicine. This advancement expands OraSure’s diagnostic portfolio, supporting clinical research and personalized healthcare through improved blood sample integrity and efficient proteomic workflows.
Key Players
Leading Companies of the Market
Becton, Dickinson and Company
Terumo Corporation
Siemens Healthineers
Abbott Laboratories
Cardinal Health, Inc.
Nipro Corporation
3M Company
Bio-Rad Laboratories
Fujifilm Corporation
BioIVT
Cenova Diagnostics
Several leading companies pursued innovation-driven growth strategies in 2025 and 2026. Siemens Healthineers, for example, leveraged digital connectivity to enable remote monitoring of blood collection processes, resulting in a reported 12% improvement in procedural efficiency. Terumo Corporation focused on regional expansion in the Asia Pacific, taking advantage of supportive government healthcare policies and expanding diagnostic infrastructure, which contributed to an approximate 14% increase in its market revenue.
Blood Collection Devices Market Future Outlook
The blood collection devices market is expected to experience strong growth in the coming years, driven by rising demand for efficient, safe, and minimally invasive diagnostic solutions. Technological innovations, including automated systems, safety-engineered needles, vacuum-assisted devices, and capillary collection solutions, will continue to enhance workflow efficiency and patient safety. Growing prevalence of chronic diseases, expansion of point-of-care testing, and the rise of home healthcare services will further boost adoption. Additionally, regulatory emphasis on biocompatibility and sustainability, along with increasing integration of advanced materials and digital connectivity, will create opportunities for product innovation and market expansion globally.
Blood Collection Devices Market Historical Analysis
The blood collection devices market has demonstrated steady growth over the past decade, driven by increasing demand from hospitals, diagnostic laboratories, and outpatient care facilities. Historically, the market has been led by vacuum blood collection systems due to their efficiency and safety, while syringes, needles, and blood bags have maintained consistent demand for specialized procedures and transfusions. Technological advancements, such as the introduction of safety-engineered needles and automated collection systems, gradually improved workflow efficiency and reduced occupational hazards. Regional growth was initially led by North America and Europe due to advanced healthcare infrastructure, while Asia Pacific emerged as a key growth region with expanding healthcare investments and rising diagnostic testing volumes.
Sources
Primary Research Interviews:
Clinical Laboratory Managers
Phlebotomists and Nurses
Hospital Procurement Officers
Blood Collection Device Manufacturers
Databases:
Global Blood Collection Devices Market Reports
Healthcare Equipment & Supplies Market Databases
Diagnostic Laboratory Market Reports
Magazines:
Medical Device & Diagnostic Industry (MD+DI)
Laboratory Equipment Magazine
Clinical Lab News
Healthcare Technology Magazine
MedTech Outlook
Journals:
Journal of Clinical Laboratory Analysis
Clinical Biochemistry
Journal of Laboratory Automation
Annals of Clinical Biochemistry
Clinical Chemistry and Laboratory Medicine
Newspapers:
The New York Times (Health)
The Guardian (Technology & Health)
Financial Times (Healthcare)
Reuters Health
Associations:
American Association of Clinical Chemistry (AACC)
International Federation of Clinical Chemistry and Laboratory Medicine (IFCC)
Clinical Laboratory Standards Institute (CLSI)
World Health Organization (WHO) – Laboratory and Diagnostics Division
European Federation of Clinical Chemistry and Laboratory Medicine (EFLM)
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients