Australia Reprocessed Medical Devices Market is estimated to be valued at USD 484.1 Mn in 2025 and is expected to reach USD 782.5 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 7.1% from 2025 to 2032. 2 Mn in 2023 and is expected to exhibit a CAGR of 7.1% during the forecast period 2025-2032.
Analysts’ Views on the Australia Reprocessed Medical Devices Market :
In June 2022, Medline ReNewal, a company that collaborated with and manages reprocessing programs for over 2,000 healthcare organizations, opened a new distribution center in Mississippi U.S. The facility serves major hospitals, nursing homes, and military facilities in the region. The company invested in expanding its storage and distribution capacity in Msississippi to meet the product needs of its healthcare customers. Over US$ 350.0 Mn in annual orders are expected to be handled from the Southaven facility.
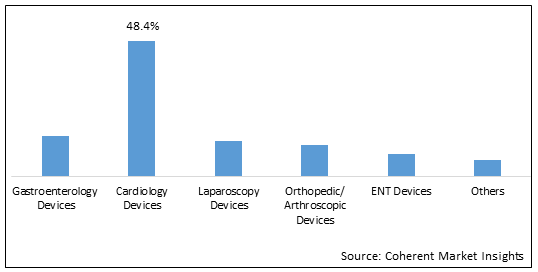
Figure 1. Australia Reprocessed Medical Devices Market Share (%), by Device, 2025
To learn more about this report, Download Free Sample
Australia Reprocessed Medical Devices Market - Driver
Partnerships, Mergers and Collaboration Scenarios
Increasing partnerships, mergers, and collaborations are a major factor leading to the high demand for Australia Reprocessed medical devices market. For instance, in September 2022, Innovative Health, a reprocessing company focused on Electrophysiology devices, and Medline ReNewal, a biopharmaceutical company, announced that they had entered into a five-year agreement to continue their collaboration on a single-use device reprocessing program for hospitals. The joint reprocessing initiative aims to improve the performance of medical device reprocessing programs, which often do not provide optimal savings results for the hospital.
Increasing Product Approvals
The increasing product launch activities to invent newer reprocessing devices or its accessories can drive growth of the Australia reprocessed medical devices market. For instance, June, 2020, NEScientific, Inc., a company that provides real-time simulation technologies that are used to support physicians in better targeting tissues during a variety of minimally invasive, announced that it had received FDA clearance for reprocessing the 014 Digital IVUS catheter.
Australia Reprocessed Medical Devices Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, the U.A.E., Egypt, and others, are facing problems with the transportation of drugs from one place to another.
However, the COVID-19 pandemic had a positive impact on the Australia reprocessed medical devices market. This is because, in Australia, heaps of single-use gowns, facemasks, face shields, aprons, gloves, goggles, sanitizers, sharps, and syringes were disposed of every day as a result of the pandemic. Moreover, the establishment of new home and hotel quarantine facilities and isolation in various Australian states and territories increased the risks of transmission of infections among people in these facilities and the likelihood of general waste becoming contaminated with medical waste.
Australia Reprocessed Medical Devices Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 484.1 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.1% | 2032 Value Projection: | USD 782.5 Mn |
| Segments covered: |
|
||
| Companies covered: |
Stryker, Medline Industries, LP, Hygia Healthcare, Cleanpart Gmb (A subsidiary of Mitsubishi Chemical Group), ReNu Medical, Inc., SureTek Medical, and NEScientific, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Australia Reprocessed Medical Devices Market Segmentation:
The Australia reprocessed medical devices market report is segmented into device, classification, and end user.
By device, the market is segmented into gastroenterology devices, cardiology devices, laparoscopy devices, orthopedic/arthroscopic devices, ENT devices, and others. Out of which, the cardiology devices segment is expected to hold a dominant position in the Australia reprocessed medical devices market during the forecast period, and this is attributed to the rising prevalence of cardiovascular diseases in Australia.
By classification, the market is segmented into critical, semi - critical, non - critical . Out of which, the non-critical segment is expected to hold a dominant position in the Australia reprocessed medical devices market during the forecast period and this is attributed to the increasing product launches in the segment.
By end user, the market is segmented into hospitals, diagnostic centers, ambulatory surgical centers. Out of which, the hospital segment is expected to dominate the market over the forecast period and this is attributed to the availability of medical devices in hospitals.
Among all the segmentations, the cardiology device segment has the highest potential due to the increasing prevalence of diseases and disorders across the world over the forecast period. For instance, according to the data shared in June 2021, by the World Health Organization (WHO), a specialized agency of the United Nations responsible for international public health, Cardiovascular Diseases (CVDs) were the leading cause of death in Australia in 2020, and an estimated 1.2 Mn lives were affected each year by heart stroke or vascular disease.
Australia Reprocessed Medical Devices Market Cross Sectional Analysis:
Among devices, the cardiology devices segment is expected to drive growth in Australia. For instance, according to the data analyzed by the Australian Institute of Health and Welfare, in 2021, Coronary Heart Disease (CHD) was the leading single cause of death in Australia, accounting for 17,300 deaths.
Australia Reprocessed Medical Devices Market : Key Developments
In July 2022, NEScientific, Inc., a company focused on reprocessing single-use peripheral vascular catheters, announced it had received FDA clearance for reprocessing the Philips Spectranetics 0.9mm OTW Turbo-Elite laser atherectomy catheter. The device is used to treat peripheral arterial disease (PAD) and emits high-energy ultraviolet light to vaporize blockages inside the vessels. This laser catheter is one of the most commonly used modalities for this common procedure, with treatments done in acute care settings, surgical centers, and the Office-based Lab (OBL).
On March 30, 2022, Solvay, a science company whose technologies bring benefits to many aspects of daily life, and Mitsubishi Chemical Advanced Materials, a global manufacturer of high-performance thermoplastic materials in the form of semi-finished products and finished parts, partnered to recycle end-of-life medical components within medical devices.
In August 2021, Stryker, a medical technology company, announced its plans for a $14 million expansion of its warehouse on Portage Road, U.S. According to a statement released by Stryker, the expansion will allow the Portage Distribution Center to operate more efficiently, allow all inventory to be stored on-site, and allow for future sales growth as well as an increased component footprint for new product launches.
Australia Reprocessed Medical Devices Market: Key Trends
Increasing product launch by market players
The introduction of quality management systems for medical devices is also expected to aid in growth of Australia reprocessed medical devices market. For instance, in April 2020, Cleanpart GmbH a industrial services company introduced a quality management system for medical devices according to DIN EN ISO 13485:2016.
The expansion of new research and development centers globally is expected to propel growth of Australia reprocessed medical devices market. In June 2022, Stryker, a global medical technology company, announced the opening of its new research and development facility in Gurgaon, India. This will help accelerate innovation in India and globally and further support the company’s mission to make healthcare better.
Australia Reprocessed Medical Devices Market: Restraint
Lack of confidence in reprocessed medical devices
Negative perceptions about reprocessed medical devices are also expected to limit market growth. It is often perceived that reprocessed medical devices are inferior to new devices in terms of safety, efficacy, and product quality. Despite evidence and studies demonstrating how reprocessed medical devices are safe and effective, negative perceptions about their usage persist.
This can be overcome by arranging seminars and workshops for healthcare professionals as well as caregivers, highlighting the safety and efficacy of reprocessed medical devices
Australia Reprocessed Medical Devices Market - Key Players
The major players operating in the Australia reprocessed medical devices market include Stryker, Medline Industries, LP, Hygia Healthcare, Cleanpart Gmb (A subsidiary of Mitsubishi Chemical Group), ReNu Medical, Inc., SureTek Medical, and NEScientific, Inc.
*Definition: Reprocessing" refers to a process carried out on a used device in order to allow its safe reuse. It includes its cleaning, disinfection, sterilization, and related procedures, as well as testing and restoring the technical and functional safety of the used device. While single-use devices are intended by the manufacturer to be used only once, reusable devices are intended by the manufacturer to be reused after appropriate procedures such as cleaning, disinfection, and sterilization have been carried out. Therefore, Medical Device Regulation (MDR) establishes different responsibilities and requirements for the reprocessing of single-use devices and reusable devices.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients