Single-use medical device reprocessing is the concept of sterilizing, repackaging, testing, and manufacturing a used product. There is no clear definition for the term single-use device and it is entirely based upon the end-user perception. Reprocessing of single-use devices is acceptable if the device is properly sterilized while keeping its physical and technological functionalities intact. Single-use medical device reprocessing is done in two ways: third party processing and in-hospital processing. Third party processing is considered to be a better option, as regulatory authorities can impose strict regulations for validation and quality assurance purpose.
Global Reprocessing and Reuse of Single Use Medical Devices Market - Impact of the Coronavirus (COVID-19) Pandemic
The coronavirus (COVID-19) outbreak was first reported on December 31, 2019, in Wuhan, China. The World Health Organization declared COVID-19 as pandemic on March 11, 2020. According to the Coronavirus (COVID-19) Weekly Epidemiological Update by the World Health Organization, December 12, 2021, the number of new COVID-19 cases and deaths globally decreased slightly in the second week of December 2021, with over 269 million cases and over 5.3 million deaths.
Impact of COVID-19 on Demand and Supply of Reprocessing and Reuse of Single Use Medical Devices
The coronavirus (COVID19) pandemic and lock down in various countries across the globe have impacted the financial status of businesses in all sectors. Private health care sector is one of the sectors, which is majorly impacted by the COVID-19 pandemic.
Moreover, coronavirus pandemic has negatively impacted the development, production, and supply of medical devices and affected growth of the medical devices businesses of various companies across the globe, as COVID-19 pandemic had led to unprecedented lockdown in several countries, globally. This lockdown has resulted in closure of industrial establishments, except manufacturing of essential commodities and disruption in supply chain of the products. According to Morulaa HealthTech Private Limited, (offers regulatory support/ product approval services for medical devices in India and other South East Asian markets) report published in December 2020, during the COVID-19 pandemic, various healthcare facilities are focusing on the reprocessing and reuse of single use medical device with the help of registered third party reprocessors in order to combat the constraints faced by the manufacturers/ healthcare facilities in the supply of single-use medical devices and also aids healthcare facilities in reducing cost and controlling the waste production of healthcare facilities.
The global reprocessing and reuse of single use medical devices market is estimated to be valued at US$ 1,843.4 Mn in 2021, and is expected to exhibit a CAGR of 10.2% over the forecast period (2021-2028).
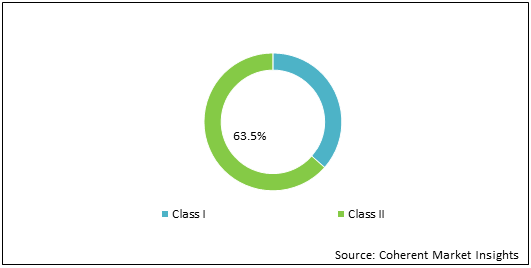
Figure 1: Global Reprocessing and Reuse of Single Use Medical Devices Market Share (%) Analysis, By Product Type, 2021
To learn more about this report, Download Free Sample
Increasing focus on reducing medical waste is the major factor that is expected to drive the market growth over the forecast period.
In order to reduce medical waste, healthcare facilities are increasingly focusing on implementing various approaches including minimizing the use of materials, reuse of instruments, and reprocessing and reuse of single use medical devices, which are expected to drive the market growth over the forecast period. According to the Association of Medical Device Reprocessors (AMDR), in 2019, approximately 15 million pounds of medical waste were reduced due to the use of reprocessed single-use medical devices.
Cost Savings Associated with Reprocessing Single Use Medical Devices
Minimally invasive technology based on single use devices (SUDs) is largely being used in modern medicine. Many SUDs used to perform surgical procedures are expensive to purchase and store, and typically require large inventories than multiple-use devices. Thus, reprocessing and reuse of SUDs become common in many health care facilities in order to reduce the cost. According to the Association of Medical Device Reprocessors (AMDR), the savings of hospitals and the surgical center partners due to third party reprocessing of SUD has increased from US$ 20 million in 1999 to US$ 471 million by 2018, in the U.S, Canada, and Europe.
Reprocessing and Reuse of Single Use Medical Devices Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2020 | Market Size in 2021: | US$ 1,843.4 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2021 to 2028 |
| Forecast Period 2021 to 2028 CAGR: | 10.2% | 2028 Value Projection: | US$ 3,644.3 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Stryker Corporation, Medline Industries, Inc., Suretek Medical, Renu Medical Inc., Johnson & Johnson, Northeast Scientific Inc., Medtronic Plc, Steripro Canada, Vanguard AG, Innovative Health, Verathon Inc., and Hogy Medical Co., Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Reprocessing and Reuse of Single Use Medical Devices Market – Restraints
Concerns about the risk of infection and injury associated with the reuse of single use medical devices are expected to hinder the marker growth over the forecast period. Following are the risks associated with the reuse of single use medical devices (SUDs).
Global Reprocessing and Reuse of Single Use Medical Devices Market – Regional Analysis
On the basis of region, the global reprocessing and reuse of single use medical devices market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa.
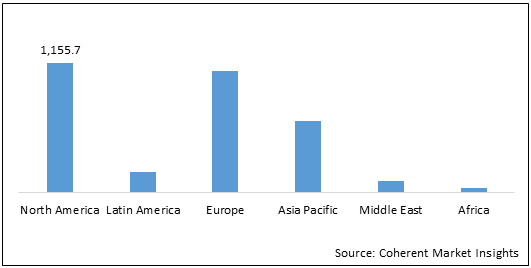
Among regions, North America is expected to hold a dominant position in the global reprocessing and reuse of single use medical devices over the forecast period, owing to healthcare facilities such as hospitals, homecare facilities, and ambulatory surgical centers adopting the reprocessing and reuse of single use medical devices, as it is cost-effective and decreases the waste of medical devices. For instance, according to the Association of Medical Device Reprocessors (AMDR) report published in 2018, almost 25% or quarterly hospitals in the U.S and Canada practice reuse and reprocessing of single use medical devices as a cost saving and waste management strategy.
Figure 2: Global Reprocessing and Reuse of Single Use Medical Devices Market Value (US$ Mn), by Region, 2021
To learn more about this report, Download Free Sample
Global Reprocessing and Reuse of Single Use Medical Devices Market – Competitive Landscape
Major players operating in the global reprocessing and reuse of single use medical devices market include Stryker Corporation, Medline Industries, Inc., Suretek Medical, Renu Medical Inc., Johnson & Johnson, Northeast Scientific Inc., Medtronic Plc, Steripro Canada, Vanguard AG, Innovative Health, Verathon Inc., and Hogy Medical Co., Ltd.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients