Automated Analyzers Market Size and Trends
Global automated analyzers market is estimated to be valued at USD 3.17 Bn in 2025 and is expected to reach USD 6.18 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 10.0% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
Global automated analyzers market is expected to witness significant growth due to increasing incidence of chronic and infectious diseases globally. Technological advancements focused on development of compact, efficient, and high-throughput automated analyzers can boost sales of these systems. Adoption of decentralized or point-of-care testing using automated analyzers is also expected to increase in the near future.
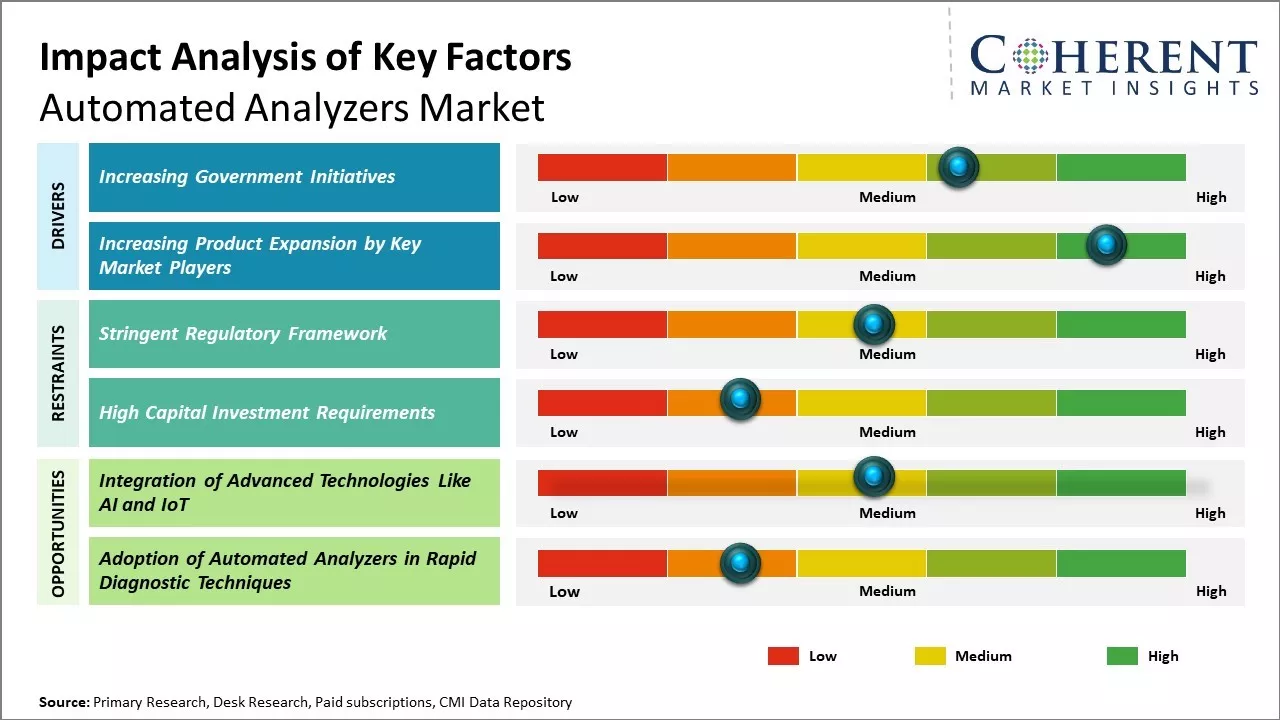
Increasing Government Initiatives
Increasing government initiatives is expected to drive the market growth over the forecast period. For instance, in November 2023, The Embassy of the Republic of Korea (RoK) donated fully automated biochemistry analyzers worth US$ 402,500 to Nepal. The official handover ceremony was held at the Ministry of Health and Population, Government of Nepal, in the presence of Dr. Roshan Pokhrel, Secretary of the Ministry of Health and Population, and Mr. Park Tae-Young, Ambassador of the Republic of Korea in Nepal. Secretary Pokhrel accepted the donation on behalf of the Nepali government.
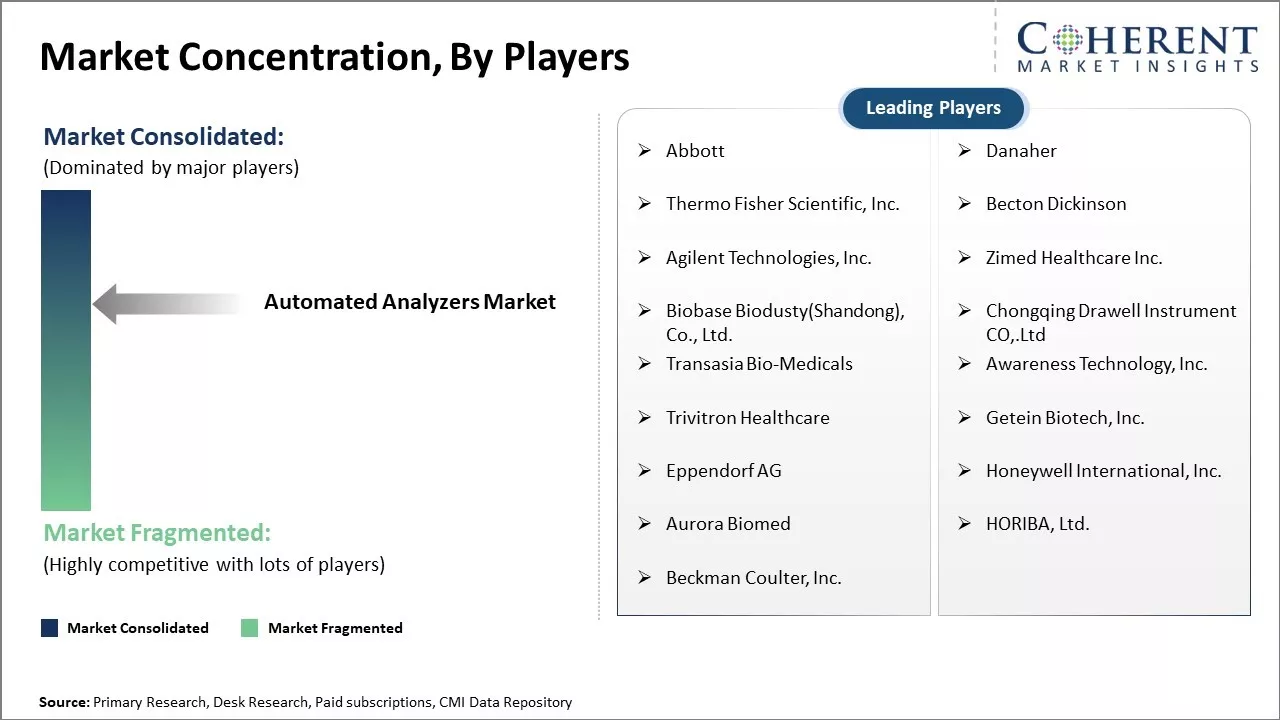
Market Concentration and Competitive Landscape
Get actionable strategies to beat competition: Download Free Sample
Increasing Product Expansion by Key Market PlayersThe key market players are focused on expanding their product portfolio to strengthen their geographic presence in the market by launching new products, and this is expected to drive the market growth. For instance, on January 29, 2024, Beckman Coulter Diagnostics, a healthcare company, announced its plan to introduce the DxC 500 AU Chemistry Analyzer, a cutting-edge automated clinical chemistry system, at the Medlab Middle East event held from February 5-8, 2024, at the Dubai Exhibition and Trade Center in the United Arab Emirates.

To learn more about this report, Download Free Sample
Market Challenge – Stringent Regulatory FrameworkStringent regulatory norms across various countries poses significant challenges for the growth of global automated analyzers market. Governments are increasingly tightening regulations to ensure patient safety and high accuracy of diagnostic test results. This leads to longer approval timelines for new products and technologies in this market. For instance, the U.S. Food and Drug Administration (FDA) subjects devices used for in-vitro diagnostic purposes to premarket approval to establish safety and effectiveness. Getting this approval involves clinical trials and extensive documentation, which can take 2-3 years. European In Vitro Diagnostic Medical Device Regulation (IVDR) 2017/746 mandates stricter regulatory measures concerning the design, production, and post-market monitoring of IVD devices, surpassing the requirements of previous directives. Compliance with these updated regulations has increased the cost and time invested by manufacturers.
Market Opportunity – Integration of Advanced Technologies Like AI and IoT
The integration of advanced technologies such as artificial intelligence and internet of things provides opportunities for the global automated analyzers market growth. These emerging technologies can drive automated operations and real-time monitoring capabilities within biochemistry analyzers. Artificial Intelligence (AI) powered algorithms and machine learning models can be leveraged to analyze complex biochemical test results more accurately. This can help clinicians arrive at diagnoses faster. The AI models can also be trained on vast amounts of historical test data to identify subtle patterns and correlations that are not apparent to humans. The incorporation of Internet of Things (IoT) sensors into biochemistry analyzers and test kits allows for round-the-clock remote monitoring of critical parameters.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
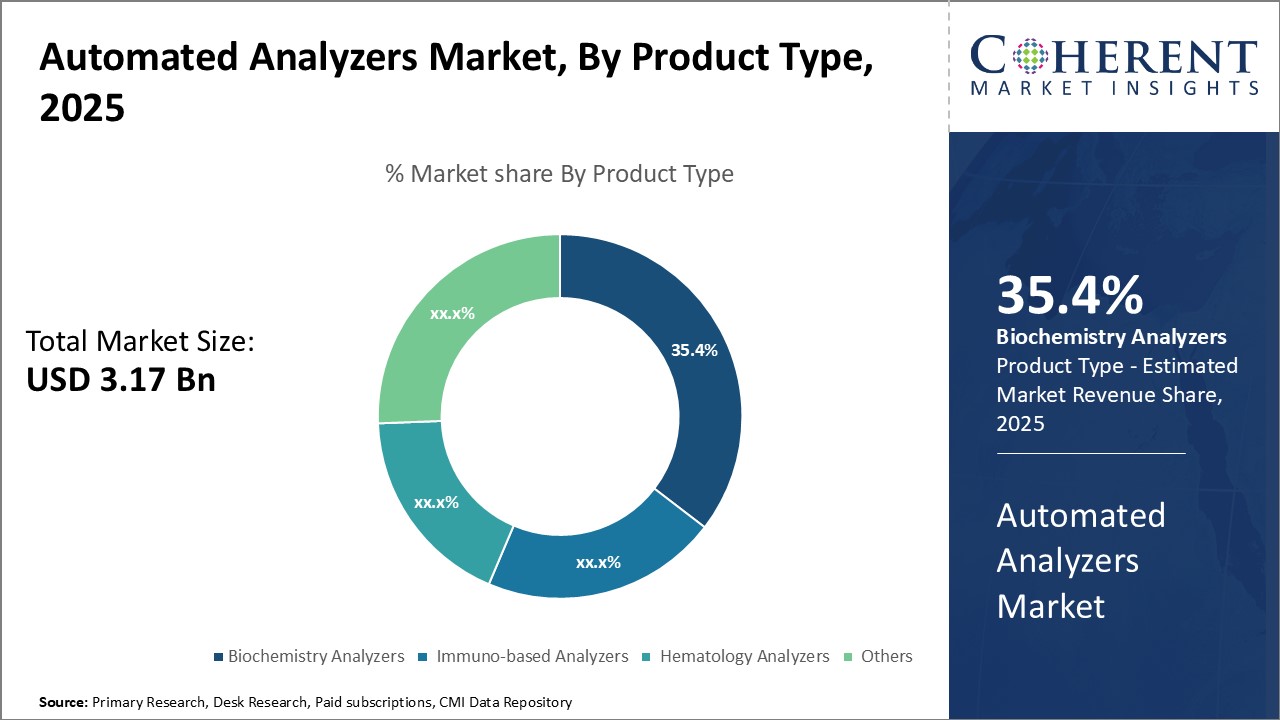
Insights, By Product Type: Automated diagnosis and analysis boosts adoption of biochemistry analyzersProduct Type segment is sub-segmented into biochemistry analyzers, immuno-based analyzers, hematology analyzers, and others. Biochemistry analyzers sub-segment is estimated to hold 35.4% of the market share in 2025, owing to their ability to perform rapid, automated diagnostic testing and analysis of various biochemical parameters. Biochemistry analyzers can assist clinicians in streamlining diagnostic workflows and improving turnaround times by automating multi-test sample analysis on a single platform. Their automated processing allows for testing of high volume routine samples without compromising on result accuracy. This alleviates workload pressures on laboratory staff and enables real-time monitoring of critical biomarkers. Moreover, biochemistry analyzers integrate various detection technologies to analyze electrolytes, proteins, metabolic panels and specialized tests from a small volume of sample. Biochemistry analyzers is the preferred solution for automating diagnostic workflows and enhancing laboratory efficiency in the global automated analyzers market.
Insights, By Sample Type: Maximizing throughput with blood sample analysis
Sample Type segment is sub-segmented into blood, urine, serum, plasma, and others. Blood sub-segment is estimated to hold 53.3% of the market share in 2025, due its widespread use in clinical diagnostics and testing. Being a versatile and accessible sample type, blood can be analyzed on automated analyzers to gain clinically relevant insights into various health conditions. Automated hematology analyzers specialized for blood sample analysis excel at maximizing throughput while maintaining accuracy. This meet rising workloads resulting from increasing disease prevalence and screening needs. Advanced capabilities like rapid cell counting, differentiation and reticulocyte analysis from small blood volumes expedite anemia screening, infection diagnostics, and monitoring of blood disorders. Growing diagnostic requirements for complete blood counts, coagulation assays, and blood chemistries boosts demand for high-volume blood analyzers. Their integration of flow cytometry, microscopy and data management solutions ensures reliable, standardized processing of large batches of blood samples round the clock. This aids continuous monitoring especially for critical care, thus, driving greater uptake of blood sample analyzers in the global automated analyzers market.
Insights, By End User: Streamlining diagnostics in diagnostic laboratories
End User segment is sub-segmented into hospitals & clinics, diagnostic laboratories, pharmaceutical and biotechnology companies, and others. Diagnostic laboratories sub-segment is estimated to hold 34.2% of the market share in 2025, due to increasing utilization of automated analyzers for streamlining complex diagnostic workflows. These can reliably process round-the-clock high volumes of samples for a wide array of pathology, clinical chemistry and immunoassay tests. This consistency augments quality of test results for clinicians. Automation increases throughput while lowering turnaround times for reports. It frees up laboratory staff from repetitive, routine tasks, allowing them to focus more on complex analyses, result interpretations, and enhancing service quality. Advanced data management and connectivity further optimize information flow within diagnostic networks. With growing pressure to rapidly scale testing capacity and improve efficiency, diagnostic laboratories are increasingly reliant on automated analyzers to streamline operations and maximize resources. Their ability to harmonize multi-faceted diagnostics in a high-paced setting positions them as the leading end user in the global automated analyzers market.
Regional Insights
Need a Different Region or Segment? Download Free Sample
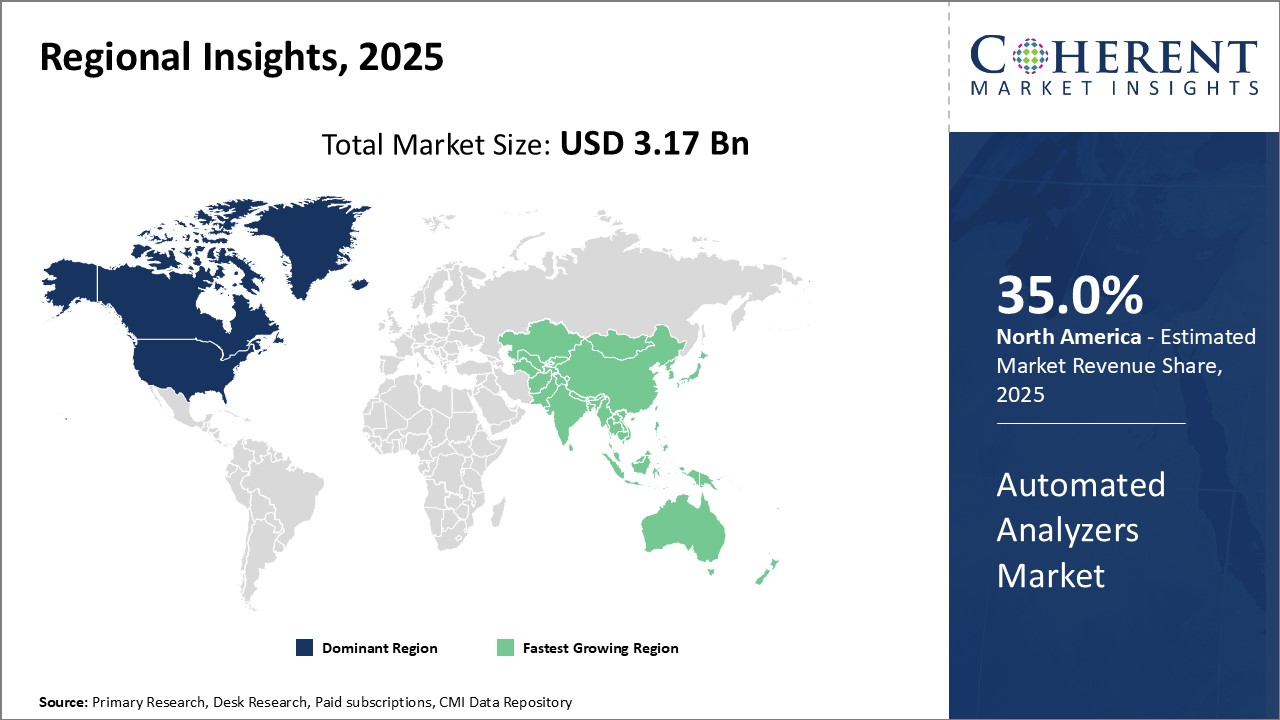
North America remains the dominant region in the global automated analyzers market, and is estimated to hold 35.0% of the market share in 2025. The presence of major players from the U.S. such as Thermo Fisher Scientific, Agilent Technologies, and Danaher has enabled them to gain significant market share in the region. These players have extensive distribution networks across countries like the U.S. and Canada that provide customers easy access to their wide range of automated analyzers products. Furthermore, the presence of a highly developed healthcare infrastructure and large healthcare expenditure in the region has boosted demand for automated analyzers in various end user segments including hospitals, clinical laboratories, biotechnology companies and research institutes. Rising healthcare awareness and focus on early disease diagnosis has boosted demand for advanced medical technologies like automated analyzers.
The automated analyzers market in Asia Pacific region has emerged as the fastest growing regional market globally. Rapidly developing economies like China and India are attracting greater investments from international medical device companies to tap into their huge untapped growth potential, and this has positively impacted the availability of advanced analyzers systems within Asia Pacific. Growing medical tourism and establishment of new clinical laboratories and research centers has boosted demand for high-precision automated equipment in the region. Favorable government policies supporting the adoption of advanced healthcare technologies can also drive the market growth.
Market Report Scope
Automated Analyzers Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 3.17 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 10.0% | 2032 Value Projection: | USD 6.18 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Abbott, Danaher, Thermo Fisher Scientific, Inc., Becton Dickinson, Agilent Technologies, Inc., Zimed Healthcare Inc., Biobase Biodusty(Shandong), Co., Ltd., Chongqing Drawell Instrument CO,.Ltd, Transasia Bio-Medicals, Awareness Technology, Inc., Trivitron Healthcare, Getein Biotech, Inc., Eppendorf AG, Honeywell International, Inc., Aurora Biomed, HORIBA, Ltd., Beckman Coulter, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Automated Analyzers Industry News
- On January 30, 2024, Beckman Coulter, Inc., one of the clinical diagnostics leaders and a subsidiary of Danaher, launched the DxC 500 AU Chemistry Analyzer, an automated clinical chemistry analyzer, at Medlab Middle East in Dubai, held on February 5-8, 2024. The DxC 500 AU Chemistry Analyzer features advanced automation technology, onboard guided workflows, and standardized reagents for use across healthcare networks.
- On January 4, 2024, HORIBA, Ltd., a medical device company, launched 2.0 high throughput automated hematology that offers a highly flexible and efficient modular hematology solution that is fully scalable with many possible configurations for mid to large-scale laboratories
- In November 2023, CANON MEDICAL SYSTEMS CORPORATION, a medical device company, held a ceremony at its Dalian plant in Liaoning province, China, to mark the domestic production of its TBA-FX8 fully automated biochemistry analyzer, which was approved for sale in China in June 2023. Equipped with V2.0 software, TBA-FX8 can achieve automatic quality control of multiple sets of reagents at the same time, and support the early warning of the reaction curve, thus, helping inspectors to quickly analyze abnormal results, and find the cause of the problem, and assess the status of the instrument.
- In November 2023, the Embassy of the Republic of Korea (RoK) donated Fully Automated Biochemistry Analyzers, valued at US$ 402,500, to Nepal. The official handover ceremony took place at the Ministry of Health and Population, Government of Nepal, in the presence of Dr. Roshan Pokhrel, Secretary of the Ministry of Health and Population of Nepal and Mr. Park Tae-Young, the Ambassador of the Republic of Korea to Nepal. Secretary Pokhrel accepted the donation on behalf of the Nepalese government.
- In July 2023, Beckman Coulter, Inc. announced that it had received U.S. Food and Drug Administration clearance for its new DxC 500 AU Chemistry Analyzer, characterized by its automation, showcases a continual dedication to advancing product innovation within the in vitro diagnostic sector
*Definition: Automated analyzers use to analyze biological samples like blood, urine, and others automatically and efficiently. These analyzers automate the delivery, analysis, and reporting of test results with minimal human involvement. These are commonly used in healthcare settings like hospitals, diagnostic labs, and research institutes to process a large number of patient samples on a daily basis to deliver quick and accurate test results for diagnoses and treatment purposes. The analyzers offered in this market vary in capabilities and functionalities.
Market Segmentation
- Product Type Insights (Revenue, USD Bn, 2020 - 2032)
- Biochemistry Analyzers
- Immuno-based Analyzers
- Hematology Analyzers
- Others
- Sample Type Insights (Revenue, USD Bn, 2020 - 2032)
- Blood
- Urine
- Serum
- Plasma
- Others
- End User Insights (Revenue, USD Bn, 2020 - 2032)
- Hospitals & Clinics
- Diagnostic Laboratories
- Pharmaceutical and Biotechnology Companies
- Others
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Abbott
- Danaher
- Thermo Fisher Scientific, Inc.
- Becton Dickinson
- Agilent Technologies, Inc.
- Zimed Healthcare Inc.
- Biobase Biodusty(Shandong), Co., Ltd.
- Chongqing Drawell Instrument CO,.Ltd
- Transasia Bio-Medicals
- Awareness Technology, Inc.
- Trivitron Healthcare
- Getein Biotech, Inc.
- Eppendorf AG
- Honeywell International, Inc.
- Aurora Biomed
- HORIBA, Ltd.
- Beckman Coulter, Inc.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
