Global self-testing market is estimated to be valued at USD 23.49 Bn in 2025 and is expected to exhibit a CAGR of 8.9% during the forecast period (2025-2032). A self-test is a test that one can carry out independently at home to identify or diagnose various diseases. Self-tests can be used as one of the many risk-reduction strategies that protect people by lowering the likelihood of viral transmission in the case of the COVID-19, in addition to vaccination, masking, and physical separation. Additionally, diabetes is diagnosed through self-testing by monitoring blood sugar levels. The quantity of sugar or glucose in the blood can be measured using a blood sugar test. Market participants are concentrating on a variety of strategies, including funding and investments, which is expected to drive market growth over the forecast period. For instance, on 22 October 2021, the U.S. Department of Health and Human Services had announced that is has invested almost half a billion dollars in dozen of the U.S. companies to expand manufacturing of COVID-19 self-test kits. In continuation, OraSure Technologies, a Pennsylvania-based company in the medical device industry was awarded US$109 million to increase domestic manufacturing capability of rapid antigen self-tests for home care testing.
Figure 1. Global Self-Testing Market Value (USD BN), by Region, 2025
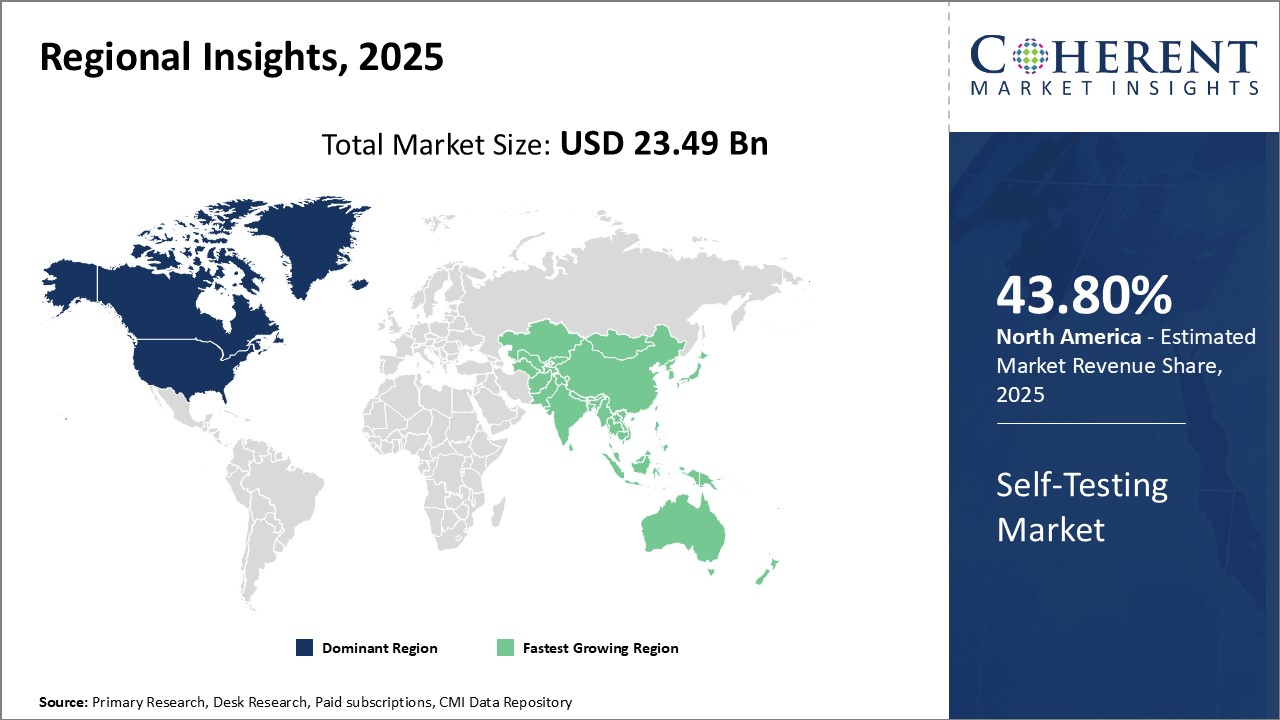
To learn more about this report, Download Free Sample
Increasing adoption of business development strategies such as agreement, collaboration, and acquisition by key market players is anticipated to drive the global self-testing market growth during the forecast period.
Due to the increase in business development strategies such collaboration and acquisition by major market players is expected to drive the self-testing market growth over the forecast period.
For instance, on 22 February 2021, Becton, Dickinson and Company a leading global medical technology company, and Scanwell Health, a leader in smartphone-enabled at-home medical tests, announced a collaboration to create an at-home rapid test for SARS-CoV-2 using a Becton, Dickinson and Company’s antigen test and the Scanwell Health mobile app.
Moreover, in April 2021, Viatris Inc., a global pharmaceutical company, and Atomo Diagnostics Limited, a global leader in the development of user-friendly rapid diagnostic test products, announced a multi-year agreement with the global health agency, Unit aid to increase access to HIV self-testing in low- and middle-income countries (LMICs).
Self-Testing Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 23.49 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.9% | 2032 Value Projection: | USD 42.67 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Becton, Dickinson and Company, Abbott, ACCESS BIO, CELLTRION INC., Siemens Healthcare GmbH, ACON Laboratories Inc., ARKRAY, Inc, F. Hoffmann-La Roche Ltd., OraSure Technologies Inc., Quest Diagnostics, Bionime Corporation, Btnx Inc., iHealth Labs Inc., InBios International, Inc. USA. And True Diagnostics Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Figure 2. Global Self-Testing Market Share, By Test Type, 2025
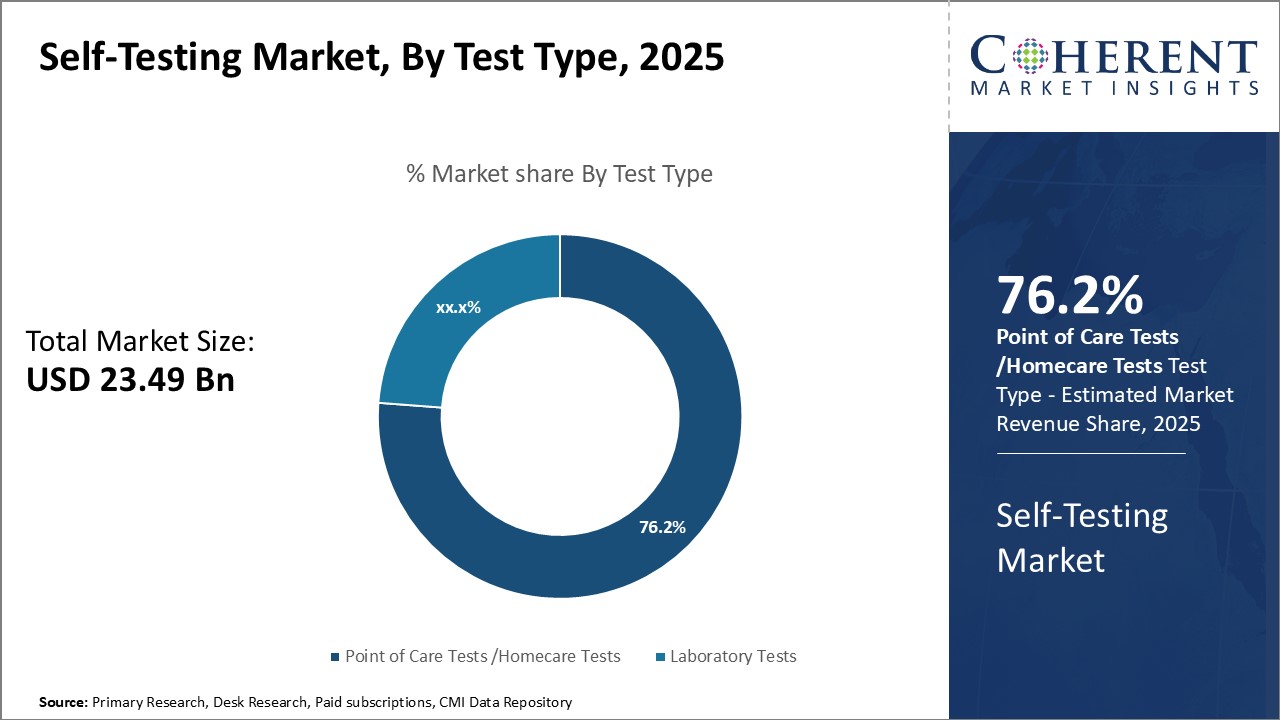
To learn more about this report, Download Free Sample
Recent Developments
In October 2023, PHASE Scientific Americas, a rapidly developing global biomedical company, announced the launch of INDICAID health, a new line of at-home health tests and digital health experiences. INDICAID health provides patients with the convenience of testing from their own homes, ensuring that they receive personalized care that aligns with their unique needs and lifestyle.
In March 2023, Lucira Health, Inc., a medical technology company, announced the nationwide launch of its breakthrough Lucira COVID-19 & Flu Home Test in the U.S., as well as the combination test's inclusion in the Australian Register of Therapeutic Goods (ARTG) for use by healthcare professionals in a point-of-care setting. The COVID-19 & Flu Home Test is the first and only combined COVID-19 & Flu test to get emergency use authorization (EUA) from the U.S. Food and Drug Administration (FDA) for over-the-counter (OTC) use at home and other non-laboratory settings.
In March 2023, Cue Health, a healthcare technology company, announced the nationwide availability of a new line of consumer-friendly at-home diagnostic test kits. These simple kits give patients access to precise, reliable testing from the comfort of their own homes, allowing them to better manage their health. The tests have been integrated into Cue Care, the company's breakthrough test-to-treat service.
In July 2022, MedAccess, a social enterprise dedicated to promoting health in low- and middle-income countries, the Clinton Health Access Initiative (CHAI), and Wondfo, a biotechnology company, announced an agreement to make Wondfo's HIV self-test available for US$ 1. The price is more than 30% lower than the current lowest-priced World Health Organization (WHO) prequalified test and 50% less than the most extensively utilized test. This makes Wondfo's HIV self-test the least expensive WHO prequalified self-test on the market.
The development of technologically advanced self-test kits is projected to propel the market growth over the forecast period.
Key market players are focusing on developing advanced technologies in self-testing kits for diagnosing severe chronic diseases such as diabetes and pregnancy detection.
For instance, in September 2021, the U.S. Patent and Trademark Office granted a U.S. patent to Gelteq Pty Ltd, a global leader in ingestible gel technology for healthcare, nutrition, and sports. It is Gelteq's first U.S. patent and it covers product technology and methods for testing human response to oral glucose loads using a proprietary gel-based delivery system. The novel diabetes diagnostic system is intended for use in pathology labs and at home.
Global Self-Testing Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 outbreak in December 2019, the disease has spread to over 100 countries across the globe. The coronavirus (COVID 19) pandemic and consequent lockdown in various countries across the globe have impacted the financial status of businesses in all sectors. Supply chain and manufacturing activities have been disrupted globally, due to lockdowns implemented by governments of countries, restricted movement, and other COVID-19 safety precautions.
Identifying individuals infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been a focal point throughout the coronavirus disease (COVID-19) pandemic. This has thus created a public health need for COVID-19 testing that is timely, reliable, and widely available.
Market players are focused on strategies such as distribution agreements for COVID-19 self-test kits. This is expected to drive the global self-testing market growth. For instance, in April 2021, Quidel Corporation, a leading provider of rapid diagnostic testing solutions, cellular-based virology assays, and molecular diagnostic systems, announced the completion of a distribution and fulfilment agreement with McKesson Corporation, a global leader in healthcare supply chain management solutions and retail pharmacy, to improve consumer access to Quidel's non-prescription QuickVue At-Home OTC (over-the-counter) COVID-19 Test.
The U.S. Food and Drug Administration (FDA) continues to monitor the supply chain. The Center for Drug Evaluation and Research (CDER) Drug Shortage Staff in 2020 asked manufacturers to evaluate their entire supply chain of various products, ethanol, EDTA and vaccines manufacturing equipment’s etc. Which in future is expected to improve the supply chain of healthcare products.
Global Self-Testing Market: Restraint
The increasing number of product recalls of self-testing kits by regulatory authorities such as the U. S. FDA, is expected to hinder the global self-testing market growth over the forecast period. For instance, in November 2021, the U.S. Food and Drug Administration (FDA) issued a recall of approximately 2 million at-home COVID-19 test kits manufactured by the Australian-based biotech company Ellume. Due to a manufacturing flaw, the test kits may produce ‘false positives’, according to the federal regulatory agency. In October 2021, the company notified the FDA about the defect in some lots. The FDA classified the Ellume test recall as ‘the most serious type’, known as a Class I recall.
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients