Xerostomia Therapeutics Market Size and Forecast – 2025 – 2032
The Global Xerostomia Therapeutics Market size is estimated to be valued at USD 1.15 billion in 2025 and is expected to reach USD 1.85 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 7.1% from 2025 to 2032.
Global Xerostomia Therapeutics Market Overview
Xerostomia therapeutics focus on relieving chronic dry mouth caused by reduced or absent saliva production. The condition is commonly associated with Sjögren’s syndrome, head and neck cancer radiotherapy, autoimmune disorders, diabetes, and long-term use of anticholinergic, antihypertensive, or psychiatric medications. Current treatments aim to restore moisture, protect oral tissues, and improve swallowing, speaking, and overall oral health. Key therapeutic categories include saliva substitutes, salivary stimulants such as pilocarpine and cevimeline, muco-adhesive gels, sugar-free lozenges, and oral moisturising sprays. These therapies help reduce dental caries, oral infections, bad breath, and discomfort linked to dry mouth.
Key Takeaways
In the product type segment, Saliva substitutes lead with 36%. Oral moisturizers grow fastest. Stimulants aid partial function, systemic agents serve autoimmune cases, while new gels and sprays expand options.
In the end user segment, Hospitals lead due to high patient volumes. Homecare grows fastest with self-care and telehealth. Specialty clinics and long-term care settings steadily expand xerostomia treatment adoption.
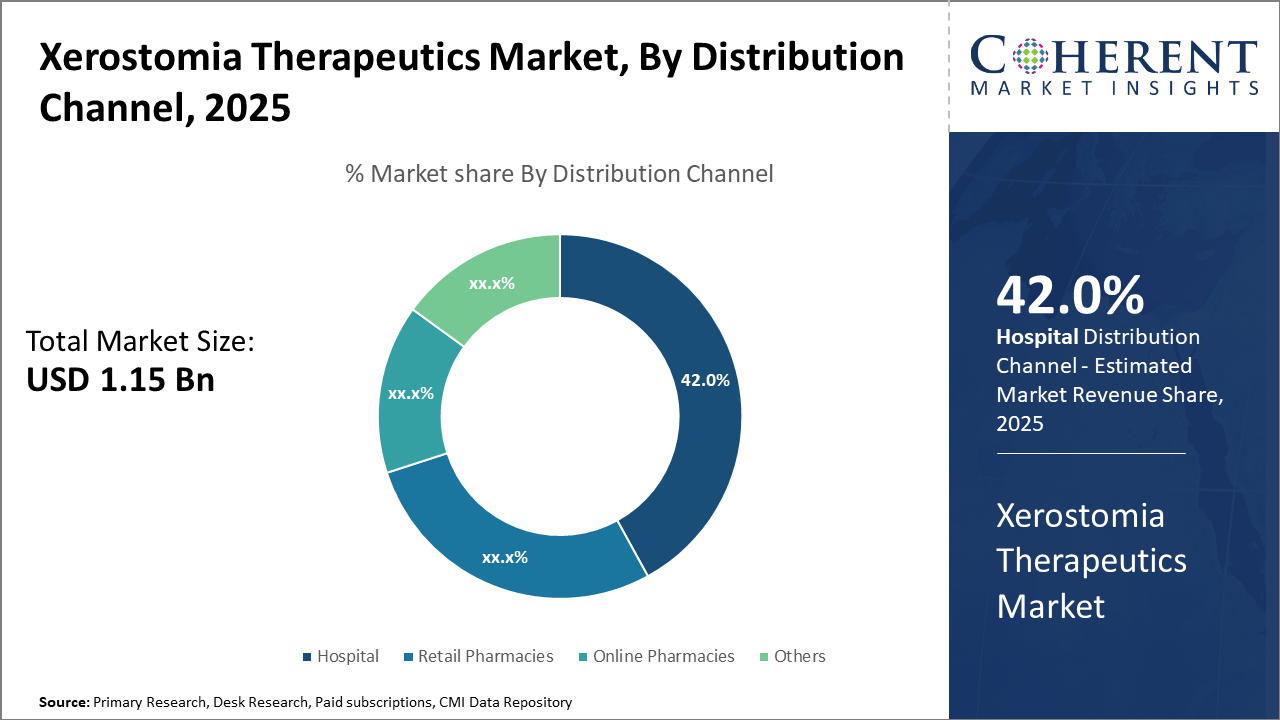
Within distribution channel, Hospital pharmacies lead with 42% via institutional demand. Online stores grow fastest. Retail pharmacies remain key, while specialty and compounding channels support customized xerostomia therapies.
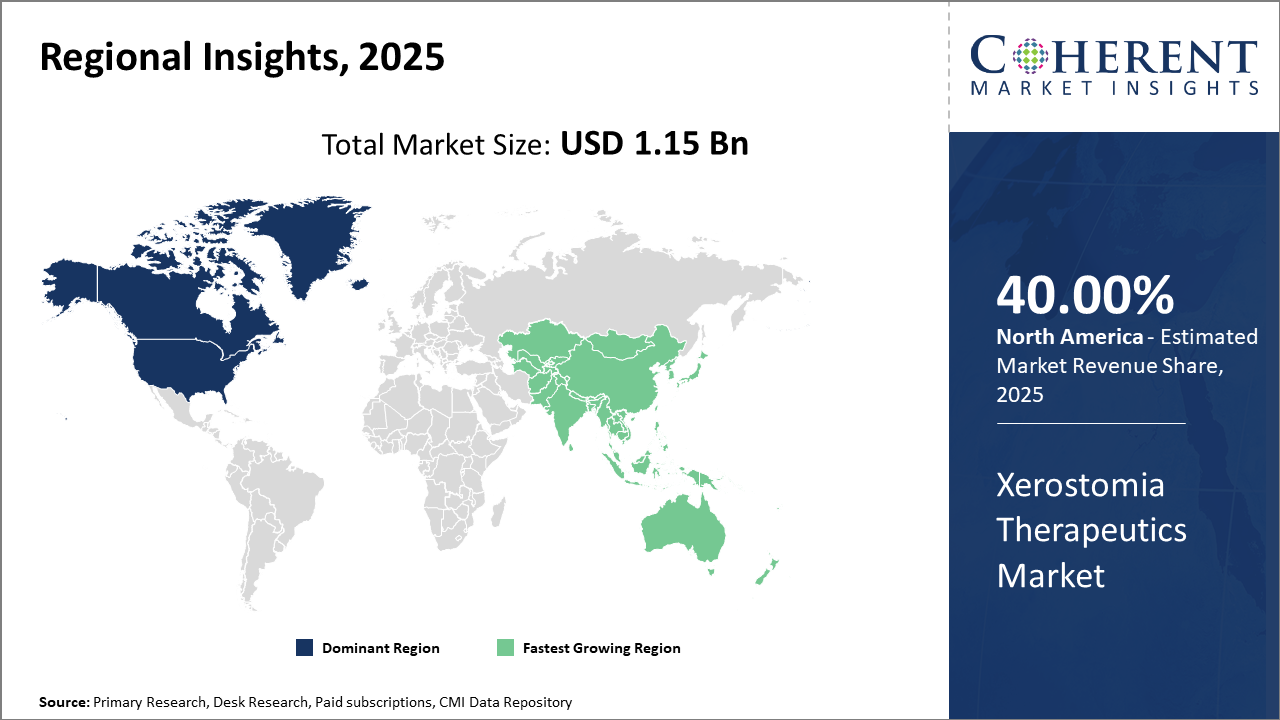
North America leads with strong infrastructure and oncology demand. Asia Pacific grows fastest at 8.5% via rising healthcare access. The U.S. drives innovation and adoption, while Japan expands through aging populations, autoimmune burden, and advanced mucoadhesive technologies.
Xerostomia Therapeutics Market Segmentation Analysis
To learn more about this report, Download Free Sample
Xerostomia Therapeutics Market Insights, By Distribution Channel
Hospital pharmacies lead the distribution channel landscape with a 42% share, supported by bulk institutional purchasing and direct alignment with treatment centers. Online stores are the fastest-growing segment, driven by rising e-commerce adoption and the demand for discreet, convenient access to xerostomia products. Retail pharmacies remain essential for over-the-counter availability, serving mild to moderate cases, while the “others” category includes specialty distributors and compounding pharmacies offering customized therapeutic options.
Xerostomia Therapeutics Market Insights, By Product Type
Saliva substitutes dominate the xerostomia therapeutics market with a 36% share, driven by rapid innovation and bioengineered formulations that closely mimic natural saliva. Oral moisturizers represent the fastest-growing subsegment due to their convenience and everyday usability. Stimulants remain valuable for patients with partial gland function, while systemic agents serve specialised autoimmune-related cases. Other emerging products, including advanced gels and sprays, enhance adherence and broaden therapeutic options.
Xerostomia Therapeutics Market Insights, By End-User
Hospitals dominate the end-user segment because they manage the highest volume of oncology and autoimmune patients who require supervised xerostomia treatment. Homecare is the fastest-growing segment, driven by rising self-care adoption and telehealth-supported chronic disease management. Specialty clinics play a strong role by expanding oral medicine services, while the “others” category—long-term care facilities and dental practices—continues to integrate xerostomia therapies into broader oral health programs.
Xerostomia Therapeutics Market Trends
The Xerostomia Therapeutics market is increasingly shaped by technological advances, with bioengineered saliva substitutes offering longer-lasting hydration and improved physiological mimicry of natural saliva.
Digital health platforms are becoming integral, enabling remote monitoring, adherence tracking, and personalised therapy adjustments for chronic xerostomia patients.
Innovations in formulation science, including sustained-release gels and muco-adhesive systems, are enhancing symptom control and reducing dosing frequency.
Emerging clinical evidence from 2024–2025 highlights superior outcomes with next-generation delivery systems, reinforcing the shift toward more effective and tolerable treatment options.
Overall, the market is moving toward patient-centric, digitally supported therapeutics that prioritise convenience, precision, and long-term quality-of-life improvement.
Xerostomia Therapeutics Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Xerostomia Therapeutics Market Analysis and Trends
North America dominates the Xerostomia Therapeutics market, supported by strong healthcare infrastructure, high patient awareness, and substantial research investments. The region accounts for about 40% of global revenue, driven by extensive oncology centers and reimbursement systems that enable easy access to therapies. Major companies like Sanofi and Takeda maintain significant operations in the region, accelerating product innovation and strengthening market penetration.
Asia Pacific Xerostomia Therapeutics Market Analysis and Trends
Asia Pacific is the fastest-growing region, recording an 8.5% CAGR driven by rising healthcare spending, broader insurance coverage, and increasing chronic disease prevalence in densely populated countries like China and India. Growth is further supported by the emergence of local pharmaceutical manufacturers developing cost-effective formulations that improve affordability and access. These factors collectively strengthen the region’s adoption of xerostomia therapies and accelerate overall business expansion.
Xerostomia Therapeutics Market Outlook for Key Countries
USA Xerostomia Therapeutics Market Analysis and Trends
The USA remains a key growth hub due to its advanced healthcare system and strong R&D investment. In 2025, over 75% of head and neck radiation patients received adjunct xerostomia therapies, generating substantial revenue. Companies are accelerating innovation, with next-generation delivery systems driving an 18% rise in patent filings compared to 2023. Widespread adoption of personalised treatment regimens further supports sustained market expansion.
Japan Xerostomia Therapeutics Market Analysis and Trends
Japan’s market is driven by its rapidly aging population and a high prevalence of autoimmune disorders that contribute to xerostomia, creating steady therapeutic demand. Supportive government policies focused on geriatric care and reimbursement for advanced treatments strengthen revenue growth. Japanese pharmaceutical firms have also led innovations in mucoadhesive technologies, securing over 15% of the regional share in Asia Pacific. Ongoing collaborations with North American and European companies further enhance Japan’s role in shaping next-generation therapeutic solutions.
Analyst Opinion
The growing patient base affected by xerostomia is a major demand-side driver, particularly among the elderly and individuals undergoing radiation therapy. In 2024, oncology center data showed that more than 70% of head and neck cancer patients receiving radiotherapy developed xerostomia, significantly increasing the need for dedicated therapeutic interventions and long-term symptom management.
On the supply side, formulation advancements such as sustained-release lozenges and bio-adhesive gels have improved treatment effectiveness and patient adherence. The launch of next-generation mucoadhesive delivery technologies in late 2023 resulted in a 15% year-on-year sales increase for leading manufacturers, highlighting strong adoption of innovative product formats.
Pricing has remained stable, with moderate adjustments aligned to rising R&D spending. Import-export records from 2024 show North America contributing nearly 45% of global exports of xerostomia therapeutic products, underscoring its robust manufacturing capacity and expanded production networks.
The market is also benefiting from wider therapeutic applications beyond oncology, particularly in autoimmune conditions such as Sjögren’s syndrome. Global clinical registries reported a 12% rise in Sjögren’s diagnoses in 2025, reinforcing strong future growth prospects and expanding patient eligibility for xerostomia treatments.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 1.15 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.1% | 2032 Value Projection: | USD 1.85 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Teva Pharmaceutical Industries Ltd., GlaxoSmithKline plc, Mylan N.V., Biotene (GSK Consumer Healthcare), Santen Pharmaceutical Co. Ltd., Ferring Pharmaceuticals, Bausch Health Companies Inc., Highland Therapeutics, Amgen Inc., Aqualief Therapeutics AB, BioXell S.A., Innovaderm Ltd., Otsuka Pharmaceutical Co.Ltd., Perrigo Company plc, Ipsen S.A., Sun Pharmaceutical Industries Ltd., Lupin Limited, Menarini Group | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Xerostomia Therapeutics Market Growth Factors
Key growth drivers in the Xerostomia Therapeutics Market include the rising global burden of cardiovascular and thrombotic diseases, where antithrombin therapy serves as a critical intervention; for example, cardiovascular surgeries in the U.S. increased by 7.1% between 2023 and 2025, directly elevating antithrombin demand. Technological innovations, such as advances in recombinant DNA technology, have significantly reduced production costs and improved product efficacy, boosting market growth in developed and emerging economies alike.
Additionally, favorable government policies supporting plasma collection and rare disease management have propelled market expansion, especially in regions such as North America and Europe. Increased awareness programs regarding congenital antithrombin deficiency contribute further by expanding patient diagnosis rates, enhancing market scope, and penetration.
Xerostomia Therapeutics Market Development
In April 2019, Advanz Pharma acquired global (excluding Japan) marketing rights to Salagen, a pilocarpine-based therapy for dry mouth in Sjögren’s syndrome and radiotherapy-induced xerostomia, to strengthen and expand its specialty care portfolio. The company also gained rights to Panretin for AIDS-related Kaposi sarcoma, supporting its renewed M&A strategy and aligning with its PLAN initiative focused on strategic product acquisitions.
In April 2024, TePe has launched its new Hydrating Mouthwash and Hydrating Mouth Gel designed to relieve dry mouth and support oral health. Developed by dental experts, the products provide long-lasting hydration, help stimulate saliva, and include fluoride protection. Trial data shows 85% of users reported symptom relief, addressing rising demand for effective dry mouth solutions.
Key Players
Leading Companies of the Market
Teva Pharmaceutical Industries Ltd.
GlaxoSmithKline plc
Mylan N.V.
Biotene (GSK Consumer Healthcare)
Santen Pharmaceutical Co., Ltd.
Ferring Pharmaceuticals
Bausch Health Companies Inc.
Highland Therapeutics
Amgen Inc.
Aqualief Therapeutics AB
BioXell S.A.
Innovaderm Ltd.
Otsuka Pharmaceutical Co., Ltd.
Perrigo Company plc
Ipsen S.A.
Sun Pharmaceutical Industries Ltd.
Lupin Limited
Menarini Group
Several market players have increasingly adopted acquisition strategies to broaden product portfolios and accelerate entry into emerging markets. For example, a key industry player completed a notable acquisition of a biotech firm focusing on mucoadhesive formulations in 2024, leading to a 14% increase in market revenue. Additionally, partnerships centered on R&D innovation, particularly in Asia Pacific, have resulted in several patented therapeutic solutions with expanded indications, enhancing competitive positioning.
Xerostomia Therapeutics Market Future Outlook
The future outlook for xerostomia therapeutics remains highly promising as demand continues to rise with increasing cancer survivorship, growing autoimmune disease diagnoses, and global population aging. Innovation will play a central role, with bioengineered saliva substitutes, next-generation mucoadhesive systems, and regenerative therapies aimed at restoring salivary gland function expected to reshape treatment standards. Digital health tools will further support remote monitoring, adherence tracking, and personalised care. Expanding homecare adoption, improved reimbursement in developing regions, and the entry of cost-efficient local manufacturers will enhance accessibility. Collectively, these factors position the market for strong, sustained growth over the next decade.
Xerostomia Therapeutics Market Historical Analysis
The historical evolution of xerostomia therapeutics reflects a shift from basic moisture-replacement products to more advanced, clinically supported solutions. Before 2015, the market centered on simple saliva substitutes and OTC moisturizers offering short-term relief. From 2016 to 2020, rising awareness of xerostomia’s impact on oral health and quality of life drove the adoption of prescription stimulants and the development of bio-adhesive gels and improved oral moisturizers. Since 2021, innovation has accelerated with enhanced formulation technologies, growing autoimmune and oncology patient populations, and broader reimbursement support. Increasing use of digital adherence tools and home-based management has further strengthened the market’s progression toward patient-centric care.
Sources
Primary Research Interviews:
Oral Medicine Specialists
Oncologists (Head & Neck Radiation)
Rheumatologists
Clinical Pharmacologists
Dental Surgeons
Databases:
ClinicalTrials.gov
PubMed Oral Health & Salivary Gland Studies
NIH Salivary Gland Biology Database
Global Health Data Exchange (GHDx)
Magazines:
Pharmaceutical Technology
Medical Device Network
Oncology Times
Dental Products Report
Journals:
Journal of Oral Pathology & Medicine
Oral Diseases
Journal of Dental Research
Associations:
American Academy of Oral Medicine (AAOM)
International Association for Dental Research (IADR)
Sjögren’s Foundation
American Dental Association (ADA)
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients