Women’s Health Diagnostic Test Market Size and Forecast – 2025 – 2032
The Women’s Health Diagnostic Test Market size is estimated to be valued at USD 6.4 billion in 2025 and is expected to reach USD 11.9 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 9.3% from 2025 to 2032.
Global Womens Health Diagnostic Test Market Overview
Women’s health diagnostic tests are specialized medical tests designed to detect, monitor, and manage health conditions that primarily affect women. These include screenings for breast cancer, cervical cancer, ovarian cancer, osteoporosis, pregnancy and fertility issues, sexually transmitted infections, and hormonal imbalances among others. These diagnostics employ advanced technologies such as imaging, molecular diagnostics, point-of-care testing, and genetic panels to provide early detection and personalized treatment options.
Key Takeaways
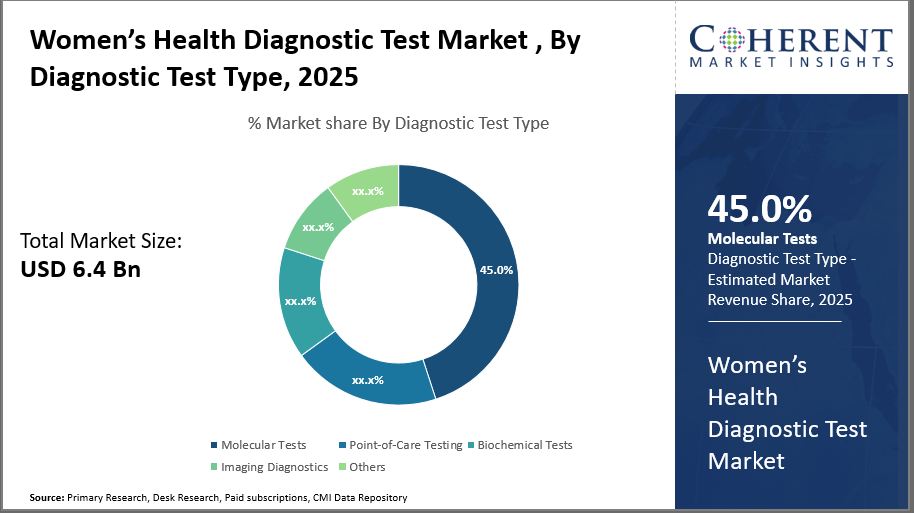
The molecular tests segment leads the market with a 45% market share, primarily driven by demand for high-sensitivity detection in oncology and reproductive health.
Hospital end-users dominate the industry share at 50%, reflecting reliance on institutional infrastructure for complex diagnostics.
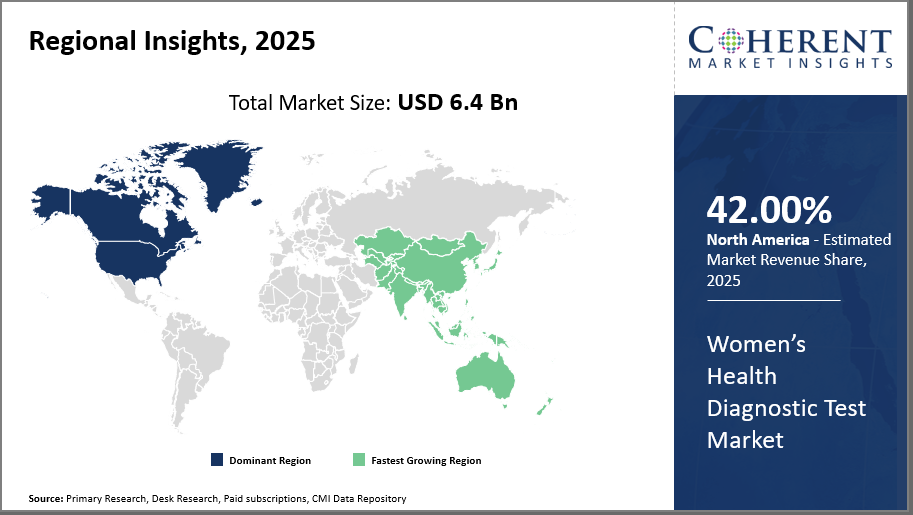
North America holds the predominant market share of approximately 42%, driven by advanced healthcare infrastructure and favorable reimbursement policies.
Asia Pacific exhibits the fastest market growth, registering a CAGR exceeding 11%, attributed to rising healthcare investments and widening access to diagnostics.
Women’s Health Diagnostic Test Market – Segmentation Analysis
To learn more about this report, Download Free Sample
Women’s Health Diagnostic Test Market Insights, By Diagnostic Test Type
In terms of diagnostic test types, Molecular Tests dominate the market share at 45%. Molecular Tests drive growth due to their high sensitivity and specificity, particularly in cancer and reproductive health diagnostics. These tests are widely used for early detection, diagnosis, and monitoring of various women-specific health conditions including cervical, breast, and ovarian cancers, as well as infectious diseases like HPV and HIV. The increasing adoption of molecular diagnostics is fueled by their high accuracy, sensitivity, and the ability to facilitate personalized treatment strategies.
Women’s Health Diagnostic Test Market Insights, By Application
In terms of application, Cancer Screening makes up 38% of the market share and is the primary growth engine due to increasing incidence of breast, cervical, and ovarian cancers. This subsegment benefits from breakthroughs in genomic and biomarker testing that enable earlier diagnosis and better prognostication. Pap smears, HPV DNA-based screening, and sophisticated imaging methods for early cancer diagnosis are important diagnostic procedures in this area. In particular, government screening initiatives and technological developments in AI-based cytology and self-sampling kits are driving the rapid growth of cervical cancer screening.
Women’s Health Diagnostic Test Market Insights, By End User
In terms of end-users, Hospitals commanding the largest market share of 50%. This dominance is driven by extensive infrastructure, skilled personnel, and advanced equipment necessary for complex diagnostic procedures. Hospitals are key providers of diagnostic services for women, offering advanced testing for cancer (such as breast, cervical, and ovarian), infectious diseases, pregnancy and fertility monitoring, and other women’s health disorders. This segment benefits from the availability of sophisticated diagnostic infrastructure and skilled healthcare professionals.
Women’s Health Diagnostic Test Market Trends
The Women’s Health Diagnostic Test market is experiencing a paradigm shift toward integration of multi-omics approaches, combining genomics, proteomics, and metabolomics for holistic disease assessment. For example, the adoption of multi-analyte blood tests for early detection of ovarian and breast cancers increased by 29% in 2024, demonstrating this accelerating trend.
Another emerging trend is the rise of wearable biosensors for continuous health monitoring, supported by collaborations between tech companies and diagnostic firms, which grew substantially in 2023-2025. Additionally, markets are witnessing a surge in demand for personalized fertility diagnostics, stimulated by demographic trends like delayed childbearing and rising infertility rates globally.
Women’s Health Diagnostic Test Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Women’s Health Diagnostic Test Market Analysis and Trends
In North America, the dominance in the Women’s Health Diagnostic Test market is supported by a mature healthcare ecosystem, significant research funding, and well-established screening programs. The region accounts for roughly 42% of the overall market share, fuelled by pioneering companies like Roche Diagnostics and Quest Diagnostics. Favorable reimbursement frameworks alongside robust government initiatives for women’s health foster sustained business growth and expanding market share.
Asia Pacific Women’s Health Diagnostic Test Market Analysis and Trends
The Asia Pacific exhibits the fastest growth at CAGR over 11%, driven by surging healthcare investments, increasing awareness, and expanding diagnostic infrastructure. Rapid urbanization and rising prevalence of women's health issues in countries such as China and India have accelerated market demand. Key players such as Abbott Laboratories and Siemens Healthineers are enhancing their footprint here through collaborations and localized manufacturing, capturing significant revenue streams.
Women’s Health Diagnostic Test Market Outlook for Key Countries
United States Women’s Health Diagnostic Test Market Analysis and Trends
The US market remains the largest single-country contributor, attributed to high healthcare expenditure and advanced health infrastructure. For instance, the implementation of widespread breast cancer screening programs has increased diagnostic test volumes by over 18% from 2023 to 2025. Prominent market companies, including Labcorp and Thermo Fisher Scientific, continue to innovate with AI-driven diagnostics and liquid biopsy technologies, cementing the country’s pivotal role in revenue generation and market expansion.
Japan Women’s Health Diagnostic Test Market Analysis and Trends
Growing awareness of women's health issues and technology improvements are driving the market for women's health diagnostic tests in Japan. The market includes diagnostic tests for a variety of ailments, including osteoporosis, pregnancy and reproductive problems, breast and cervical cancer, and hormone abnormalities. Genetic testing and biomarker analysis are two examples of non-invasive, miniature diagnostic tools that are becoming more and more popular since they allow for early detection and individualized care.
Analyst Opinion
Demand-side dynamics highlight the growing adoption of non-invasive diagnostic tests, which accounted for approximately 62% of the total market revenue in 2024. Consumer preference for early disease detection without surgical intervention strongly drives growth, evident from a 15% year-on-year increase in home-based testing kits across North America in 2025.
Supply chains are adapting swiftly, with production capacity expansions in molecular diagnostics facilities notably rising by 20% in Asia Pacific between 2023 and 2024. This augmentation aligns with the tripling of exports of key reagents used in women’s health diagnostics by major industry hubs in China, supporting substantial market growth.
Market prices for advanced genomic testing have decreased by nearly 12% in 2024 due to process optimizations and economies of scale, enhancing affordability. This has expanded use cases from oncology diagnostics into conditions like preeclampsia and polycystic ovary syndrome (PCOS), significantly broadening market scope.
Across micro and nano analytical devices segments, innovations witnessed a 30% CAGR in patent filings between 2022 and 2025. Integration of AI algorithms in diagnostic platforms propelled accuracy improvements, driving a surge in market penetration, especially in telehealth applications post-pandemic.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 6.4 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.3% | 2032 Value Projection: | USD 11.9 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Roche Diagnostics, Abbott Laboratories, Siemens Healthineers, Becton, Dickinson and Company, Hologic, Inc., Quest Diagnostics, Labcorp, PerkinElmer, BioMérieux, Thermo Fisher Scientific. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Women’s Health Diagnostic Test Market Growth factors
The expansion of government-supported health screening programs, particularly targeting cervical and breast cancer, remains a critical growth driver. Countries such as the U.S. and Germany have increased funding by over 25% for women’s health diagnostics in the past two years, bolstering market revenue.
Rising incidence of lifestyle-related diseases, coupled with increasing healthcare spending, particularly in Asia Pacific, is fuelling demand for sophisticated diagnostic modalities. Technological breakthroughs such as liquid biopsies and AI-powered imaging diagnostics have improved test accuracy and reduced diagnosis time, attracting increased hospital and laboratory adoption. The growing consumer trend toward personalized healthcare and home-testing solutions is reshaping the market landscape, with home-based testing sales growing 18% annually in North America as of 2024.
Women’s Health Diagnostic Test Market Development
In September 2025, Salient Bio, a UK-based diagnostics firm, has closed a £2.35 million seed investment round led by THENA Capital to support the commercial launch of their inflammatory bowel disease diagnostic test based on the microbiome. In order to expedite research into illnesses that disproportionately impact women, the business intends to employ its own SIGNAL platform.
In May 2025, Wisp, the biggest pure play women's telehealth provider in the United States, debuted its diagnostics vertical. Wisp serves over 1.5 million clients with sexual and reproductive health solutions. With the help of the Wisp At-Home Testing & Follow-Up Care service, patients may obtain easy at-home test kits for a range of medical ailments, allowing them to gather samples and obtain findings without physically visiting a clinic.
In May 2025, The first diagnostic platform designed just for women, Femcliffe, was introduced by Redcliffe Labs in an innovative attempt to address the long-standing gap in women's preventive healthcare in India. The program, which was launched as a part of Redcliffe's Mother's Day "She the Women" campaign, is a timely call to prioritize the health of women. Femcliffe offers preventive, curative, and specialized testing that is suited to women's changing health needs, as well as complete diagnostic support at every stage of life.
Key Players
Roche Diagnostics
Abbott Laboratories
Siemens Healthineers
Becton, Dickinson and Company
Hologic, Inc.
Labcorp
PerkinElmer
BioMérieux
Thermo Fisher Scientific
Through strategic alliances and acquisitions, a number of companies have significantly improved their competitive positioning by diversifying their molecular diagnostics portfolios. For example, in 2023, Roche Diagnostics acquired sequencing technology companies to expand its market reach and take the lead in advanced genomic testing. Abbott Laboratories, however, concentrated on creating portable diagnostic platforms, which helped it enter emerging markets and helped it achieve a 12% increase in sales in 2024.
Women’s Health Diagnostic Test Market Future Outlook
The Women’s Health Diagnostic Test Market will be poised for significant growth driven by evolving demographic trends, increasing clinical awareness, and consumer-led healthcare. The market will transition from traditional lab-based diagnostics to more patient-centric solutions, fueling demand in key segments such as fertility, cancer, and hormonal testing. Innovation will be propelled by the integration of advanced technologies like artificial intelligence, mobile health platforms, and non-invasive testing methods, which will enhance accessibility and accuracy. The rise of digital health applications will empower women to monitor their health proactively and access diagnostics with greater convenience.
Additionally, the expansion of healthcare infrastructure globally and increased focus on underserved populations will present ample opportunities for market players. The future landscape will be shaped by continued advancements in point-of-care testing, hybrid care models, and tailored screening protocols, fostering improved patient outcomes and market penetration worldwide. Industry leaders will likely concentrate on delivering integrated, technology-driven diagnostic solutions that emphasize personalized care and early disease detection. This dynamic environment will position the Women's Health Diagnostic Test Market for robust growth and innovation in the coming years.
Historical Development
In May 2019, Qiagen N.V. launched the first U.S. FDA-approved companion diagnostic test, Therascreen PIK3CA Kit, for identifying breast cancer patients.
In January 2019, Nurx Inc. launched At-Home HPV Test Kits to detect the types of human papillomavirus (HPV) that were commonly found in cervical cancer.
In September 2018, Mira, a part of Quanovate Tech Inc, launched the Mira Fertility system that personalized cycle prediction by measuring fertility hormone concentrations in urine samples. The devices also helped women to know their fertility status.
In December 2017, Inito, a medical technology startup, launched a fertility monitor that helped women to track their ovulation days at home in an easy and convenient way. The device enabled smartphones to perform lab-grade medical diagnostic tests at home using a single portable device.
In September 2017, GE Healthcare received U.S. FDA 510 (k) clearance for its first-ever mammography system. The device allowed women to manage their own compression during the mammography exam with the help of the company’s proprietary Senographe Pristina Dueta, a remote control.
Sources
Primary Research interviews:
Fertility Specialists & IVF Clinicians
Pathologists & Lab Technicians
Radiologists (Mammography & Imaging Experts)
Databases:
EMBASE
ClinicalTrials.gov
WHO Global Health Observatory
Magazines:
Nature Outlook (Women’s Health editions)
Medical Device & Diagnostic Industry (MD+DI)
PharmaTimes
Journals:
Breast Cancer Research
Fertility and Sterility
Journal of Clinical Oncology
Newspapers:
The Washington Post – Health & Science
The Hindu – Health
The Times of India – Health
Associations:
Centers for Disease Control and Prevention (CDC) – Women’s Health
European Society of Gynecology
Society of Women’s Health Research (SWHR)
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients