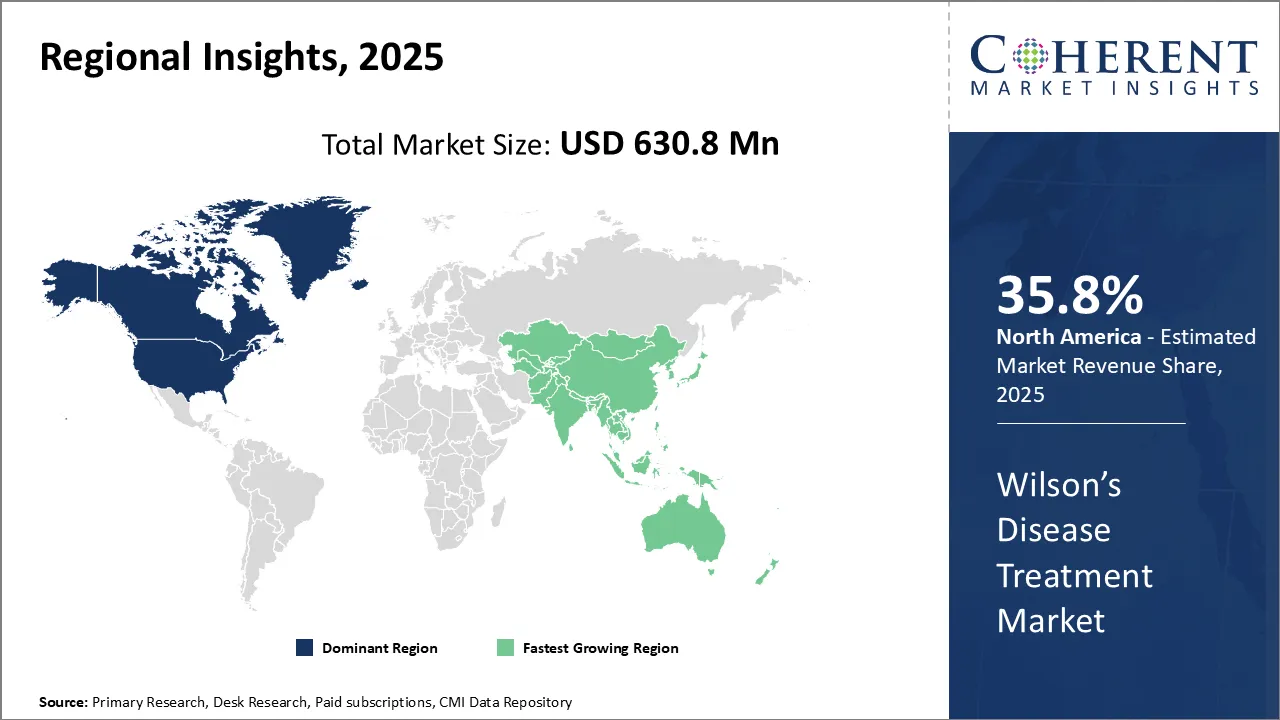
The Global Wilson's Disease Treatment Market is estimated to be valued at USD 591.2 Mn in 2025 and is expected to reach USD 993.2 Mn by 2032, exhibiting a compound annual growth rate (CAGR) of 6.7% from 2025 to 2032.
Key Takeaways of the Wilson’s Disease Treatment Market:
Market Overview:
This can be attributed to growing awareness regarding Wilson's disease and its available treatment options. Moreover, rising research and development activities focused on developing novel treatment alternatives is also expected to support the market growth during this period. Several pharmaceutical companies are investing in clinical trials to develop new drug molecules or improvement in the existing treatment regimens to offer more efficacy and fewer side effects. For instance, new chelating agents are under development that will have better tolerability and require less frequent dosing. All these factors together will boost the adoption of Wilson's disease treatment, thus driving the market during the forecast period.
Type of Treatment Insights - Increased efficacy drives the dominance of pharmacological treatment
In terms of type of treatment, the pharmacological treatment segment is expected to contribute the highest share of the market with 43.6% in 2025 owing to its increased efficacy in managing Wilson's disease symptoms compared to other treatment types. Pharmacological treatments mainly involve the use of penicillamine, trientine, and zinc which are known to be highly effective at reducing copper buildup in the body. Penicillamine remains a first-line treatment option as it directly binds with copper in blood and facilitates its excretion through urine. However, some patients often develop side effects from penicillamine such as fever, skin rashes, and joint pain which has led to higher adoption of trientine. Trientine is a copper-chelating drug that shows fewer side effects than penicillamine, thereby improving patient compliance during the treatment course. Additionally, zinc therapy has emerged as an alternative first-line option to penicillamine owing to its excellent tolerability and affordability. Zinc effectively reduces intestinal copper absorption and promotes its biliary excretion from the liver.
Route of Administration Insights - Oral administration is favored for ease and convenience
In terms of route of administration, the oral segment is expected to contribute the highest share with 43.7% in 2025 to the global Wilson's disease treatment market due to greater patient preference for oral medications over other options owing to convenience and ease-of-use factors. With oral administration, medications can be simply swallowed as tablets which does not require injection or infusion setups like the parenteral route. This provides independence to patients as oral treatment can be self-administered without any medical supervision or need to visit hospitals regularly. Additionally, oral drugs achieve better compliance levels among patients as they do not cause distress from needles or infusion ports.
Age Group Insights - Pediatric patients represent substantial opportunity
In terms of age group, the pediatric segment is expected to contribute the highest share to the global Wilson's disease treatment market with a share of 34.7% in 2025 owing to a sizeable diagnosed pediatric Wilson's disease population and effective existing treatment alternatives. Wilson's disease can manifest from a very young age and early diagnosis and treatment is crucial to prevent irreversible organ damage. At present, treatment approaches like penicillamine, zinc, and supportive dietary modifications work well for pediatric Wilson's disease. However, as children have a longer life expectancy post-diagnosis, medication challenges from long-term use and potential non-adherence issues during adolescence present key focus areas.
Need a Different Region or Segment? Download Free Sample
North America Wilson’s Disease Treatment Market Trends
In North America, the dominance in the Wilson's disease treatment market with a share of 35.8% in 2025 can be attributed to factors such as the strong presence of leading pharmaceutical companies, rising research and development activities, and favorable government policies supporting drug development.
Asia Pacific Wilson’s Disease Treatment Market Trends
The Asia Pacific region is experiencing rapid growth with a share of 26% in 2025 due to its large patient population, rising healthcare expenditure, thriving medical tourism, and the emergence of regional generic drug manufacturers. Countries like India, China, and Thailand are driving this expansion with affordable treatments, improved healthcare infrastructure, and growing demand for advanced medical solutions, making the region a key player in the global healthcare market.
Wilson's Disease Treatment Market Outlook for Key Countries
United States Wilson’s Disease Treatment Market Trends
The U.S. is witnessing significant advancements in gene therapy for Wilson's disease. In September 2023, UC Davis Health researchers administered the first-ever gene therapy for a patient with Wilson's disease as part of the CYPRUS2+ clinical trial, which showed positive results for UX701, an investigational gene therapy treatment.
Germany Wilson’s Disease Treatment Market Trends
Germany is a significant player in the Europe market for Wilson’s disease treatment. The country benefits from a robust healthcare system that supports innovative treatment options. In January 2023, the German Federal Joint Committee updated guidelines to include non-invasive testing methods for Wilson’s Disease, enhancing early diagnosis and treatment accessibility
Japan Wilson’s Disease Treatment Market Trends
The market in Japan is driven by its aging population, which increases the prevalence of genetic disorders like Wilson’s Disease. In March 2023, the Japanese Ministry of Health reported a rise in genetic testing initiatives, promoting awareness and early intervention strategies for Wilson’s Disease, which is crucial for improving patient outcomes
U.K. Wilson’s Disease Treatment Market Trends
The U.K. is focusing on integrating Wilson’s disease treatments into standard healthcare practices. In February 2023, the National Health Service (NHS) announced funding for advanced diagnostic tools and treatments for rare diseases, including Wilson’s Disease, which is expected to improve patient access to necessary therapies.
Get actionable strategies to beat competition: Download Free Sample
Key Developments:
Top Strategies Followed by Global Wilson’s Disease Treatment Market Players
Emerging Startups - Wilson’s Disease Treatment Industry Ecosystem
Wilson's Disease Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | US$ 630.8 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.7% | 2032 Value Projection: | US$ 993.2 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
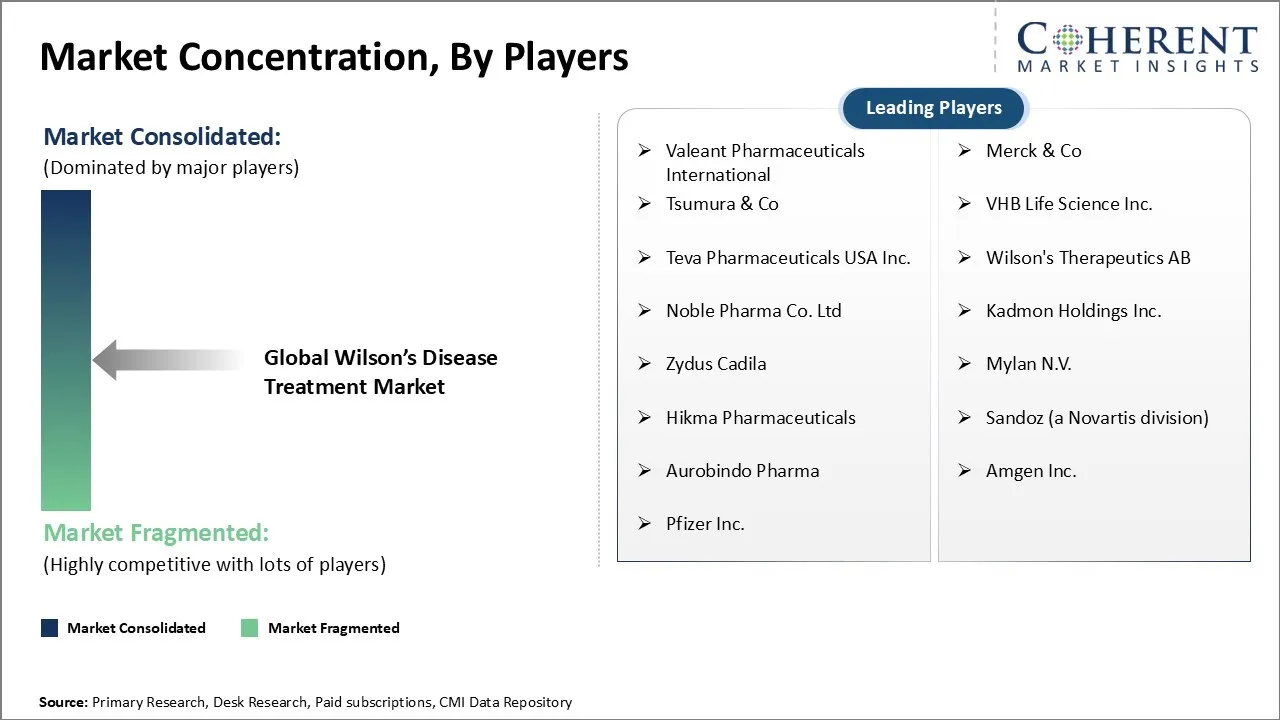
Valeant Pharmaceuticals International, Merck & Co, Tsumura & Co, VHB Life Science Inc., Teva Pharmaceuticals USA Inc., Wilson's Therapeutics AB, Noble Pharma Co. Ltd, Kadmon Holdings Inc., Zydus Cadila, Mylan N.V., Hikma Pharmaceuticals, Sandoz (a Novartis division), Aurobindo Pharma, Amgen Inc., and Pfizer Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Discover market dynamics shaping the industry: Download Free Sample
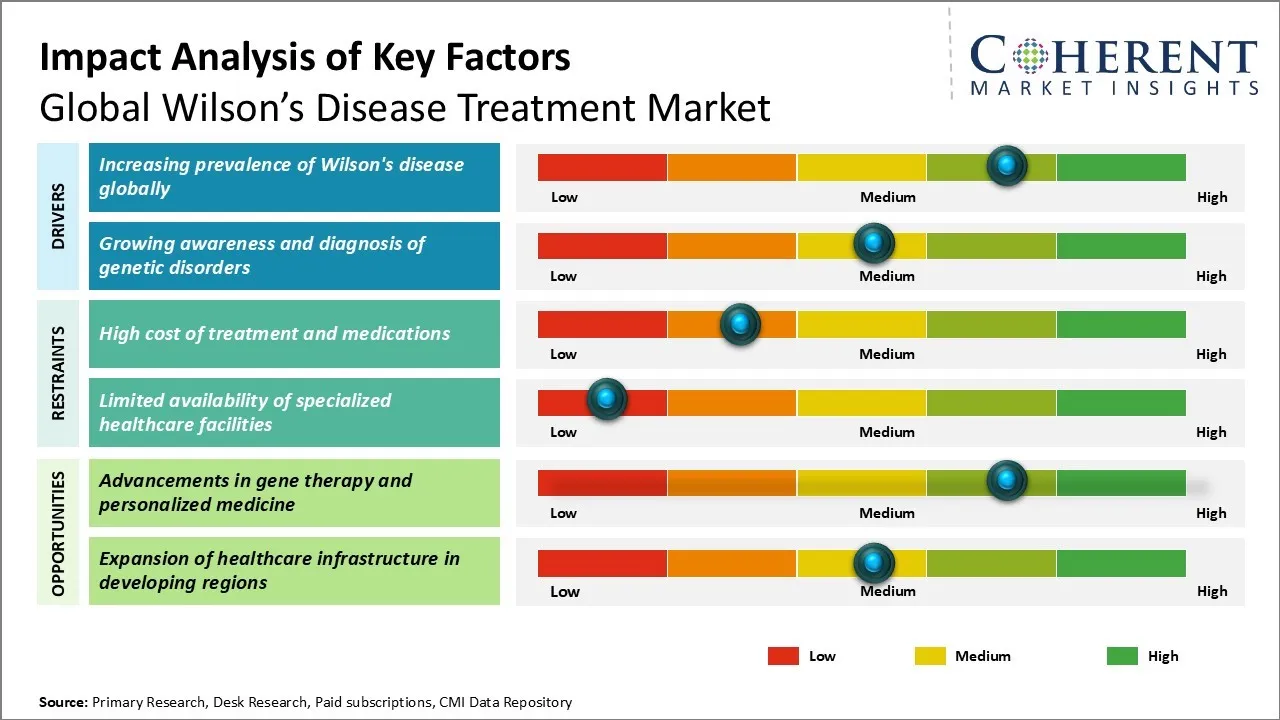
Market Driver - Increasing prevalence of Wilson's disease globally
Wilson's disease is a rare disorder that causes excess copper to accumulate in the liver and other vital organs.The prevalence of Wilson’s disease is on the rise globally as more effective diagnostic methods are helping detect previously undiagnosed cases. Countries in Europe have traditionally had higher rates but detection is improving in other parts of the world too. For instance, recent regional studies from India and parts of Africa have found higher than expected cases being identified, indicating the rates may be comparable worldwide once cases are picked up. For instance, according to the National Library of Medicine, in February 2020, the prevalence of Wilson's disease, originally estimated in 1984 as 1:30,000–1:50,000, remains valid based on updated clinical and genetic studies. Recent nationwide clinical studies estimate prevalence between 1:29,000 and 1:40,000, while genetic studies suggest it may be 3–4 times higher, raising questions about 100% penetrance.
Market Challenge - High cost of treatment and medications
One of the major challenges faced by the global Wilson's disease treatment market is the high cost associated with treatment and medications. Wilson's disease treatments often require lifelong medication regimens and monitoring to manage the accumulation of copper in the body. The medications prescribed for treatment such as penicillamine, trientine, and zinc are often very expensive and not affordable for many patients. The cost of such treatments poses a significant barrier for patients, especially in developing nations with low healthcare budgets. Furthermore, the costs associated with regular doctor visits, diagnostic tests, and other ancillary expenses involved in managing the condition, also contribute to the overall high cost of care.
Market Opportunity - Advancements in Gene Therapy and Personalized Medicine
One of the key opportunities for the global Wilson's disease treatment market lies in the advancements being made in the areas of gene therapy and personalized medicine approaches. Researchers are making progress in developing gene therapy as a potential cure for Wilson's disease. Gene therapy involves inserting a healthy copy of the gene (ATP7B) into patients' cells in order to restore the defective copper transport function. Such an approach can overcome the challenges associated with lifelong medication reliance. Similarly, advancements are also being made in using personalized medicine approaches by developing treatments tailored to an individual patient's genetic profile and disease characteristics. This novel targeted approach has the potential to optimize outcomes while reducing costs.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients