Von Willebrand Disease Treatment Market Size and Forecast – 2026 – 2033
The Global Von Willebrand Disease Treatment Market size is estimated to be valued at USD 1.8 billion in 2026 and is expected to reach USD 3.25 billion by 2033, exhibiting a compound annual growth rate (CAGR) of 8.9% from 2026 to 2033.
Global Von Willebrand Disease Treatment Market Overview
The global Von Willebrand Disease (VWD) Treatment Market is witnessing significant growth, driven by rising disease awareness, improved diagnostics, and advancements in therapeutic options. Treatments include desmopressin, plasma-derived and recombinant von Willebrand factor products, targeting prevention and management of bleeding episodes. North America leads the market due to advanced healthcare infrastructure, while Asia-Pacific shows rapid growth with expanding healthcare access. Challenges include high treatment costs and under-diagnosis in some regions. Emerging therapies, personalized medicine, and gene therapy developments are expected to further transform the market, offering improved efficacy and patient outcomes.
Key Takeaways
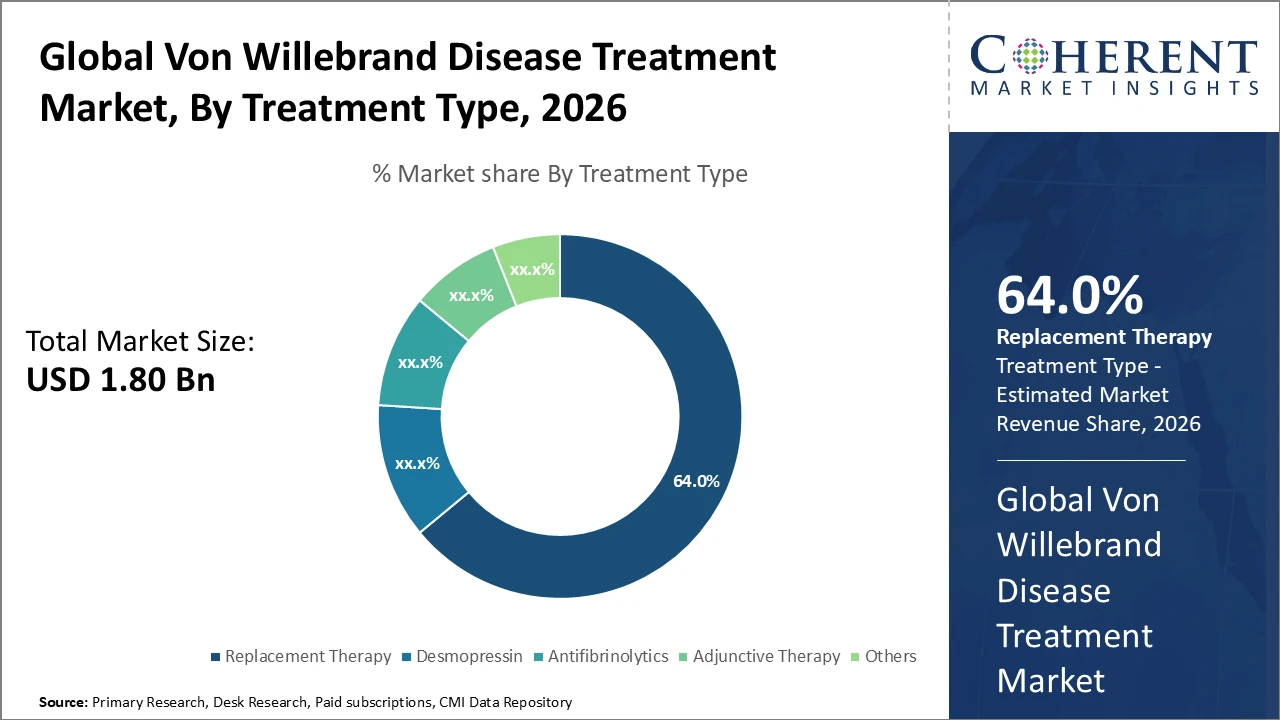
• The Replacement Therapy segment dominates the Von Willebrand Disease Treatment market, accounting for 64% of industry revenue, driven by its established efficacy and widespread patient adoption.
• Type 1 Von Willebrand Disease is the largest disease subtype, with innovations focusing on mild to moderate cases to reduce bleeding complications.
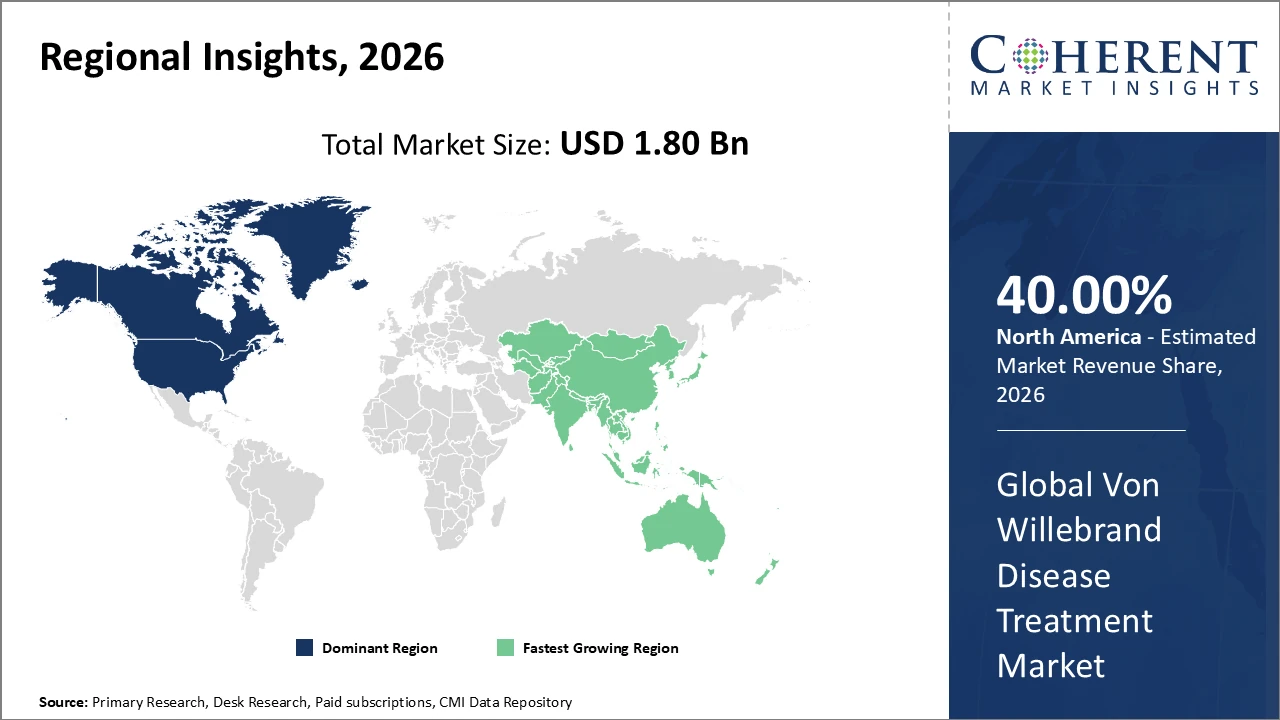
• North America holds the highest market share, supported by advanced healthcare infrastructure and strong reimbursement frameworks, contributing over 40% of total market revenue.
• Asia Pacific is the fastest-growing region, propelled by expanding healthcare access and government initiatives, with a CAGR exceeding 10% through 2033.
Von Willebrand Disease Treatment Market Segmentation Analysis
To learn more about this report, Download Free Sample
Von Willebrand Disease Treatment Market Insights, By Treatment Type
Replacement Therapy dominates the market with 64% share, driven by its established clinical efficacy and widespread use in moderate to severe Von Willebrand disease cases, providing immediate correction of deficient or defective von Willebrand factor. Desmopressin, commonly used in mild Type 1 cases, is the fastest-growing subsegment due to its non-invasive administration and increasing off-label use in pediatric patients for pre-surgical management. Antifibrinolytics are employed as adjunct treatments to enhance clot stability, mainly in dental and minor surgical procedures, while adjunctive therapies and other treatments focus on symptomatic relief and supportive care but remain niche.
Von Willebrand Disease Treatment Market Insights, By Disease Type
Type 1 Von Willebrand Disease remains the dominant subsegment due to its higher global prevalence, accounting for the majority of diagnosed cases and driving treatment demand mainly in the mild to moderate spectrum. Type 3, although rare, is experiencing accelerated growth thanks to advancements in intensive replacement therapies and emerging gene therapy candidates for severe deficiency cases. Type 2, which includes various qualitative defects, shows moderate growth primarily in developed markets with access to subtype-specific diagnostics. Acquired Von Willebrand Syndrome, often secondary to other conditions, represents a smaller but emerging subsegment due to increasing awareness and improved diagnostic capabilities.
Von Willebrand Disease Treatment Market Insights, By End-User
Hospitals dominate the market, leveraging comprehensive diagnostic and therapeutic services and accounting for the majority of revenue. Hemophilia Treatment Centers serve as critical specialized hubs offering multidisciplinary care and represent the fastest-growing segment, driven by the rise of patient-centric care models. Clinics primarily provide outpatient services for routine management, while Home Care Settings are rapidly gaining traction due to improved patient-led self-infusion therapies and telehealth integration, expanding treatment access beyond traditional facilities. Other settings, such as long-term care institutions, offer supportive services for ongoing patient management.
Von Willebrand Disease Treatment Market Trends
• The Von Willebrand Disease Treatment market is shifting towards gene therapy and personalized medicine, with several gene-editing trials initiated in 2025 showing promising early efficacy and safety results.
• Digital health integration, through mobile apps and wearable devices, enables real-time patient monitoring and improves treatment adherence.
• Home-based infusion therapies have gained momentum, especially post-pandemic, reducing healthcare facility visits and supported by telemedicine growth of 40% in 2024.
• Increasing emphasis on biosimilars aims to make treatment more accessible and cost-effective in emerging economies, with biosimilar launches expected to drive 18% market growth in Asia Pacific through 2026.
Von Willebrand Disease Treatment Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Von Willebrand Disease Treatment Market Analysis and Trends
In North America, the Von Willebrand Disease Treatment market is dominated by well-established healthcare ecosystems, supportive reimbursement policies for rare disease treatments, and a high concentration of leading companies such as CSL Behring and Pfizer. The region contributed over 40% of total market revenue in 2026, driven by advanced diagnostics and comprehensive care centers. Proactive approval pathways by the U.S. FDA have accelerated the launch of novel therapies, further fostering competitive dynamics and innovation in the market.
Asia Pacific Von Willebrand Disease Treatment Market Analysis and Trends
The Asia Pacific region exhibits the fastest growth in the Von Willebrand Disease Treatment market, with a CAGR exceeding 10%, driven by rising public health investments and growing awareness of bleeding disorders. Countries such as China and India have expanded healthcare infrastructure and reimbursement coverage, resulting in a year-on-year increase in treated patient populations of over 12% in 2026. The entry of biosimilars and generics has further lowered treatment costs, enhancing market accessibility and adoption across the region.
Von Willebrand Disease Treatment Market Outlook for Key Countries
USA Von Willebrand Disease Treatment Market Analysis and Trends
The USA represents the largest single-country contributor to the Von Willebrand Disease Treatment market, supported by robust healthcare infrastructure and government initiatives focused on rare blood disorders. In 2026, the country reported revenues exceeding USD 750 million, driven by the adoption of recombinant therapies and ongoing gene therapy clinical trials led by companies such as Takeda and CSL Behring. The expansion of hemophilia treatment centers, along with insurance policies improving drug affordability, has significantly fuelled market growth, establishing the U.S. as a global benchmark for patient care.
Germany Von Willebrand Disease Treatment Market Analysis and Trends
Germany’s Von Willebrand Disease Treatment market benefits from a well-established public health system and integrated rare disease registries that support early diagnosis and treatment. Companies such as Grifols and Octapharma have leveraged their local manufacturing presence to capture a significant market share, aided by government reimbursement programs. Strategic collaborations in research and development have accelerated the launch of next-generation therapies, contributing to approximately 15% of European market revenue in 2026 and underscoring Germany’s pivotal role in the region.
Analyst Opinion
• Increasing production capacity of recombinant von Willebrand factor products is driving market growth; in 2025, a major manufacturer raised output by 35%, resulting in a 20% price reduction and improved accessibility in emerging markets.
• Imports of specialized concentrate therapies surged by 28% in 2024, reflecting higher demand in regions with limited local manufacturing, including several European countries supported by enhanced reimbursement frameworks.
• Diversification of treatment use cases, especially in pediatric and perioperative settings, has expanded penetration; prophylactic regimens in children rose by 22% in 2026, improving patient quality of life and reducing hospitalizations.
• Growth of specialized coagulation disorder clinics contributed positively to market expansion; approximately 150 new comprehensive care centers in North America between 2024 and 2026 increased early diagnosis rates by 15%, broadening the potential treatment population.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 1.8 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 8.9% | 2033 Value Projection: | USD 3.25 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | CSL Behring, Pfizer Inc., Novo Nordisk A/S, LFB S.A., Grifols, S.A., BioProducts Laboratories, Sanofi S.A., Bioverativ Inc., Shire, Hematech Inc., Stevanato Group | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Von Willebrand Disease Treatment Market Growth Factors
Key drivers of the Von Willebrand Disease Treatment market include advancements in molecular diagnostics that enable precise patient stratification, fueling the adoption of targeted therapies. For instance, the introduction of next-generation diagnostic assays in 2025 led to a 25% increase in accurate identification of VWD subtypes. Growing government funding and reimbursement policies for rare disorders have further accelerated market growth, with the U.S. government allocating an additional USD 120 million in 2024 for bleeding disorder treatment programs. Rapid urbanization and improved healthcare infrastructure in the Asia Pacific region have opened new growth opportunities, as countries like India and China recorded annual patient treatment growth rates exceeding 12% in 2026. Rising patient advocacy and awareness campaigns have also contributed, evidenced by a 15% annual increase in specialist consultations in 2024.
Von Willebrand Disease Treatment Market Development
In September 2025, the U.S. FDA expanded approval for Takeda’s VONVENDI, a recombinant von Willebrand factor therapy, broadening its use for routine prophylaxis in adults with Type 1 and Type 2 VWD, as well as on-demand and perioperative care for pediatric patients. This approval makes VONVENDI the only recombinant VWF therapy approved for both adult and child use, enhancing treatment options and providing greater flexibility for clinicians and patients.
In January 2025, Star Therapeutics’ VGA039 received Fast Track designation from the FDA and is progressing toward Phase 3 trials as a potential subcutaneous, once-monthly preventive therapy. If approved, it could significantly reduce the infusion burden for patients, marking an important step in patient-centric care. Meanwhile, multiple companies, including Vega Therapeutics and Hemab, are advancing novel therapies such as antibody-based and subcutaneous agents aimed at boosting VWF levels and improving bleeding control, highlighting strong pipeline momentum.
Key Players
Leading Companies of the Market
CSL Behring
Pfizer Inc.
Novo Nordisk A/S
LFB S.A.
Grifols, S.A.
BioProducts Laboratory
Sanofi S.A.
Bioverativ Inc.
Shire
Hematech, Inc.
Stevanato Group
Competitive strategies in the Von Willebrand Disease Treatment market focus heavily on research and development partnerships. For example, Takeda’s collaboration with biotech firms on next-generation recombinant therapies has significantly reduced development timelines, leading to regulatory approvals in 2025. Similarly, CSL Behring’s expansion of manufacturing capabilities in the U.S. resulted in a 30% increase in market share within North America, driven by enhanced supply reliability and improved pricing competitiveness. These strategic initiatives highlight the emphasis on innovation, operational scale, and market responsiveness to maintain a competitive edge.
Von Willebrand Disease Treatment Market Future Outlook
The Von Willebrand Disease Treatment market is poised for sustained growth over the coming decade, driven by advances in gene therapy, personalized medicine, and next-generation recombinant products. Expanding diagnostic capabilities and increasing adoption of prophylactic treatment regimens are expected to improve patient outcomes and broaden the addressable population. Emerging markets, particularly in Asia Pacific and Latin America, are likely to witness strong uptake due to expanding healthcare infrastructure, government support, and greater access to biosimilars. Technological integration, including telemedicine and digital monitoring tools, will facilitate home-based care, reduce treatment burden, and enhance adherence. Overall, the market is set to evolve toward more patient-centric, cost-effective, and precision-driven therapies, with innovative pipeline products and regulatory support fueling long-term growth.
Von Willebrand Disease Treatment Market Historical Analysis
The Von Willebrand Disease Treatment market has grown steadily over the past decade, driven by increasing disease awareness, better diagnostic tools, and the introduction of recombinant and plasma-derived therapies. Initially, treatment focused on desmopressin and plasma concentrates for mild to moderate Type 1 cases and emergency bleeding episodes. Over time, prophylactic regimens and advanced replacement therapies expanded care for severe Type 2 and Type 3 patients. North America and Europe historically led the market due to strong healthcare infrastructure and reimbursement support, while emerging regions lagged. Between 2015 and 2023, the shift toward recombinant products, early gene therapy research, and patient-centric care set the stage for recent innovation and market expansion.
Sources
Primary Research Interviews:
Hematologists
Coagulation Disorder Specialists
Rare Disease Pharmacists
Clinical Trial Investigators in Bleeding Disorders
Patient Advocacy Group Representatives
Databases:
Orphanet Rare Disease Database
Global Health Data Exchange (GHDx)
WHO Global Health Observatory
National Institutes of Health (NIH) ClinicalTrials.gov
Magazines:
Hemaware
Pharmaceutical Executive
Life Sciences Leader
Rare Disease Report
Fierce Pharma
Journals:
Haemophilia
Journal of Thrombosis and Haemostasis
Blood (American Society of Hematology)
Orphanet Journal of Rare Diseases
Newspapers:
The New York Times (Health)
The Guardian (Health)
Financial Times (Healthcare)
Reuters Health
Associations:
World Federation of Hemophilia (WFH)
National Hemophilia Foundation (NHF)
International Society on Thrombosis and Haemostasis (ISTH)
European Haemophilia Consortium (EHC)
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients