Visceral Pain Treatment Market is estimated to be valued at USD 16.09 Bn in 2025 and is expected to reach USD 23.72 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 5.7% from 2025 to 2032.
Analysts’ Views on the Global Visceral Pain Treatment Market:
Visceral pain is the pain feel from internal organs such as stomach, bladder, uterus, or rectum. It can feel sharp, dull, or aching. It may be constant or come and go. This type of pain is caused by medical conditions that produce inflammation, pressure, or an injury (nociceptive pain). Pelvic pain caused by a bladder infection and abdominal pain caused by Irritable Bowel Syndrome (IBS) are examples of visceral pain. Visceral pain accounts for 28% of cancer-related pain. It is often accompanied by other pains such as neuropathic or somatic pain. Visceral pain in cancer patients may also be the result of treatment complications or comorbid diseases. Causes of cancer-related visceral pain include hepatic metastases with extension to the hepatic capsule, biliary obstruction, pancreatitis as well as pancreatic primaries and peripancreatic nodal enlargement, retroperitoneal adenopathy from metastases, and visceral organ obstruction such as small bowel or colon obstruction, mesenteric infiltration, and peritoneal implants of cancer.
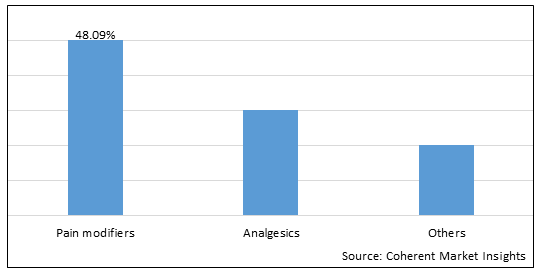
Figure 1. Global Visceral Pain Treatment Market Share (%), By Type, 2025
To learn more about this report, Download Free Sample
Global Visceral Pain Treatment Market - Drivers
Increasing new strategies to treat visceral pain
Increasing new strategies to treat visceral pain is expected to drive the global visceral pain treatment market growth over the forecast period. For instance, on July 16, 2021, Multidisciplinary Digital Publishing Institute, based in Switzerland explained that the use of tetrodotoxin (TTX) is a deadly neurotoxin produced by bacteria such as Shewanella putrefaciens., and it is a selective sodium Na+ channel blocker that has generated considerable interest in recent years. TTX is ingested by pufferfish, different marine bivalves and gastropods, and the trophic chain is thought to be the main source of its accumulation. Before the toxin had been identified, fugu fish were used in traditional Japanese medicine to cure neuralgia in leprosy patients. Subsequently, TTX was discovered, extracted and purified and used to dampen spasms in patients with tetanus. This toxin dramatically dampens the firing of abdominal pain by specifically blocking the voltage-gated sodium channels pore, thereby disrupting the passage of Na+. As such, TTX has been proposed as a possible strategy to manage pain by blocking voltage-gated sodium channels.
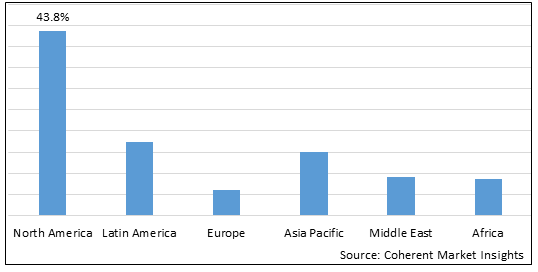
Figure 2. Global Visceral Pain Treatment Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
Global Visceral Pain Treatment Market - Regional Analysis
Among region, North America is estimated to hold a dominant position in the global visceral pain treatment market over the forecast period, owing to increasing research and development activities. For instance, on August 17, 2025, Samsung Bioepis, a South Korea-based biopharmaceutical company, and Organon, a U.S.-based pharmaceutical company, announced the U.S. Food and Drug Administration approval of Hadlima (adalimumab-bwwd) citrate-free, high concentration (100 mg/mL) injection, biosimilar to AbbVie’s Humira (adalimumab). Humira share the following indications: Rheumatoid Arthritis (RA), Juvenile Idiopathic Arthritis (JIA), Psoriatic Arthritis (PsA), Ankylosing Spondylitis (AS), adult and pediatric Crohn’s Disease (CD), Ulcerative Colitis (UC), and Plaque Psoriasis (PsO).
Global Visceral Pain Treatment Market - Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization (WHO) declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, the U.A.E., Egypt, and others, faced problems regarding the transportation of drugs from one place to another.
However, the COVID-19 pandemic had a negative impact on the global visceral pain treatment market, due to increased number of patients during the pandemic. For instance, according to an article published by the U.S. based Thomas Jefferson University, researchers used molecular tools to delete GUCY2C receptors from neuropods in mice, but not from other intestinal cells. The altered animals experienced spontaneous visceral pain that the drug linaclotide did not relieve; meanwhile water secretion was unaffected.
Visceral Pain Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 16.09 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.7% | 2032 Value Projection: | USD 23.72 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Addex Therapeutics Ltd., AstraZeneca, Astellas Pharma Inc., Allergan, Chromocell Corporation, Takeda, GIcare Pharma Inc., Abbvie, Grunenthal GmbH, Johnson & Johnson, Neurim Pharmaceuticals Ltd, and Medestea Research & Production S.p.A. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Visceral Pain Treatment Market Segmentation:
The global visceral pain treatment market report is segmented into drug type, by indication, by distribution channel, and by region.
Based on drug type, the global visceral pain treatment market is segmented into pain modifiers, analgesics, others. Out of which, analgesics segment is expected to dominate the market due to easy use of drugs and easy and quick administration process for treatment of visceral pain.
Based on indication, the global visceral pain treatment market is segmented into chronic prostatitis, interstitial cystitis, crohn’s disease, Irritable Bowel Syndrome. Out of which, irritable bowel syndrome segment is expected to dominate the market due to easy method of indication and immediate administration of treatment.
Based on distribution channel, the global visceral pain treatment market is segmented into hospital pharmacy, retail pharmacy, online channels. Out of which, hospital pharmacy segment is expected to dominate the market due to easy availability of medications.
Based on region, the global visceral pain treatment market is segmented into North America, Latin America, Europe, Asia Pacific, and Middle East and Africa. Among these, North America segment is expected to dominate the market over the forecast period due to increasing research and development activities in North America region.
Global Visceral Pain Treatment Market Cross Sectional Analysis:
Increasing treatment for visceral pain in Europe is expected to drive the growth of the drug type segment in the region. For instance, on February 1, 2023, British Medical Journal published an article explaining chronic primary pain is a recent diagnostic classification that encompasses a large number of conditions, such as fibromyalgia, complex regional pain syndrome, orofacial pain, visceral pain (eg, irritable bowel syndrome, bladder pain). Chronic pain can be difficult to treat, and management is often suboptimal - for example, the efficacy of the most common non-opioid drug treatment, paracetamol (acetaminophen), is unknown for most pain conditions. Non-steroidal anti-inflammatory drugs may provide small benefits for pain reduction in some conditions, but they need to be used with caution and for short periods because of the risk of serious adverse events with longer term use. About a third of people with chronic non-cancer related pain are prescribed opioid analgesics. The benefits of such drugs for chronic pain are, however, limited, and the potential harms outweigh the small benefits. Antidepressants are frequently used for the treatment of chronic pain.
Global Visceral Pain Treatment Market: Key Developments
On February 1, 2022, AbbVie, a U.S.-based pharmaceutical company, announced that it will continue in research in inflammatory bowel diseases with new analyses on HUMIRA (adalimumab) and the investigational uses of risankizumab (SKYRIZI) and upadacitinib (RINVOQ) at the 17th Congress of European Crohn's and Colitis Organisation (ECCO). Achievement of steroid-free remission in patients with moderately to severely active Crohn's disease during treatment with risankizumab digital oral presentation. Normalization of biomarkers and improvement in clinical outcomes in patients with Crohn's disease treated with risankizumab in the Phase 3.
On October 26, 2020, Samsung Bioepis Co., Ltd. announced results from two real-world studies of RENFLEXIS (infliximab-abda) in patients with Inflammatory Bowel Disease (IBD) registered in the U.S. Veteran Affairs Healthcare System database. The data were presented for the first time at the American College of Gastroenterology (ACG) 2020 Annual Scientific Meeting, which is held virtually from October 23 to 28, 2020.
On January 27, 2020, AstraZeneca, a pharmaceutical company announced the recovery for the global rights to brazikumab (formerly MEDI2070), a monoclonal antibody targeting IL23, from Allergan. Brazikumab is in a Phase IIb/III programme in Crohn’s disease (CD) and a Phase IIb trial in ulcerative colitis. Brazikumab is a monoclonal antibody that binds to the IL23 receptor and is in development for CD and UC with a companion biomarker. Brazikumab selectively blocks the IL23 immune signal, preventing intestinal inflammation. In Phase II trials, it demonstrated a clinical effect at week eight in tumour necrosis factor-resistant CD patients.
On February 18, 2021 Crohn's & Colitis Foundation, based in the U.K., explained the integration of mind-body treatment models into conventional healthcare is becoming the norm and not the exception, including for the treatment of chronic visceral pain. The brain-gut axis is a two-way superhighway; now recognize that the central nervous system (located in the brain and spinal cord) is constantly communicating with the messengers to and from the peripheral nervous system throughout the body. So, treatments that focus on the mind (including hypnosis, cognitive behavioral therapy, biofeedback, and mindfulness) are increasingly used to alter the way process pain messages.
Global Visceral Pain Treatment Market: Key Trends
Increasing treatment for visceral pain
Increasing treatment for visceral pain can drive growth of market. For instance, on February 1, 2023, British Medical Journal published an article explaining chronic primary pain is a recent diagnostic classification that encompasses a large number of conditions such as fibromyalgia, complex regional pain syndrome, orofacial pain, and visceral pain (e.g., irritable bowel syndrome, bladder pain). Chronic pain can be difficult to treat, and management is often suboptimal - for example, the efficacy of the most common non-opioid drug treatment, paracetamol (acetaminophen), is unknown for most pain conditions. Non-steroidal anti-inflammatory drugs may provide small benefits for pain reduction in some conditions, but they need to be used with caution and for short periods because of the risk of serious adverse events with longer term use. About a third of people with chronic non-cancer related pain are prescribed opioid analgesics. The benefits of such drugs for chronic pain are, however, limited, and the potential harms outweigh the small benefits. Antidepressants are frequently used for the treatment of chronic pain.
Global Visceral Pain Treatment Market: Restraints
Complications in the treatment of visceral pain
The complications in treatment of visceral pain is expected to hamper the global visceral pain treatment market growth. For instance, on November 30, 2022 according to an article published by American Academy of Physical Medicine and Rehabilitation, visceral pain, by definition of its local ambiguity, clouds identification of pain and can be challenging to accurately diagnose these patients. For patients without evident organic findings behavioral and psychological comorbidities diagnosis may be even more difficult. Often complicated by a behavioral or psychological diagnosis one cannot rule out concomitant somatoform or neuropathy (dysautonomia). Therefore, epidemiology, medical, interventional, rehabilitation, and psychological treatment are very challenging.
To counterbalance this restraint, more diagnostics should be introduced for the treatment of visceral pain.
Global Visceral Pain Treatment Market - Key Players
The major players operating in the global visceral pain treatment market include Addex Therapeutics Ltd., AstraZeneca, Astellas Pharma Inc., Allergan, Chromocell Corporation, Takeda, GIcare Pharma Inc., Abbvie, Grunenthal GmbH, Johnson & Johnson, Neurim Pharmaceuticals Ltd, and Medestea Research & Production S.p.A.
*Definition: Visceral pain is a type of pain related to the internal organs, which is perceived in the midline of the body. Unlike somatic pain - pain that occurs in tissues such as the muscles, skin, or joints - visceral pain is often vague, happens every so often, and feels like a deep ache or pressure.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients