Viral Vector And Plasmid Dna Testing Services Market is estimated to be valued at USD 370.4 Mn in 2025 and is expected to reach USD 1,846.9 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 25.8% from 2025 to 2032.
Analysts’ Views on Global Viral Vector and Plasmid DNA Testing Services Market:
Increasing expansion of geographical presence by the market players by opening new manufacturing center is expected to foster the grot hog global viral vector and plasmid DNA testing services market over the forecast period. For instance, in July 2022, Charles River Laboratories International, a pharmaceutical company, announced the opening of its High Quality (HQ) Plasmid DNA manufacturing Centre of Excellence at Bruntwood SciTech's Alderley Park in Cheshire, U.K. This expansion will further help the company in strengthening their geographical footprints in the global market.
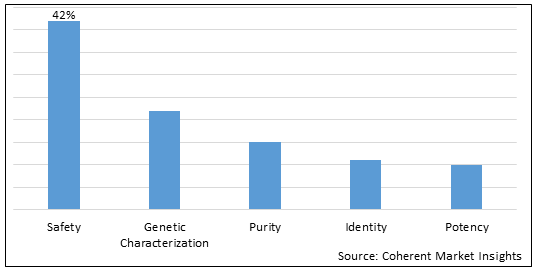
Figure 1. Global Viral Vector and Plasmid DNA Testing Services Market Share (%), By Service Type, 2025
To learn more about this report, Download Free Sample
Global Viral Vector and Plasmid DNA Testing Services Market– Drivers
Increasing number of inorganic growth strategies such as partnership and others
Increasing adoption of inorganic growth strategies such as collaboration, partnership and others by the key market players is expected to fuel the segmental growth of the over the forecast period. For instance, in October 2022, Charles River Laboratories International, Inc., a pharmaceutical company and Nanoscope Therapeutics, Inc., a clinical-stage biotechnology company developing gene therapies for retinal degenerative diseases, announced a comprehensive manufacturing collaboration utilizing Charles River's extensive contract development and manufacturing (CDMO) services in both plasmid DNA and viral vectors.
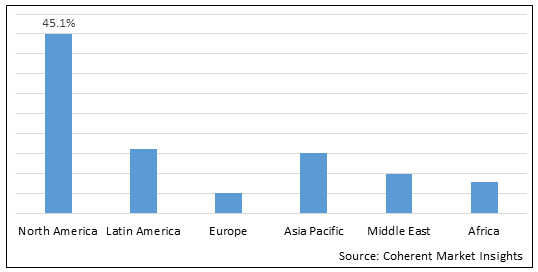
Figure 2. Global Viral Vector and Plasmid DNA Testing Services Market Value (US$ Million), By Region, 2025
To learn more about this report, Download Free Sample
Global Viral Vector and Plasmid DNA Testing Services Market- Regional Analysis
Among region, North America is estimated to hold a dominant position in the global viral vector and plasmid DNA testing services market over the forecast period, the presence of key players in the region who are focused on facility expansions to increase production and research & development activities. For instance, in October 10, 2025, VGXI Inc., a specialized contract developer and manufacturer (CDMO), announced an opening of its new headquarters and manufacturing facility located at Deison Technology Park in Conroe, Texas. U.S. for developing the next generation of gene therapies and vaccines
Global Viral Vector and Plasmid DNA Testing Services Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others faced problems regarding transportation of drugs from one place to another.
However, the COVID-19 pandemic had a positive impact on the global viral vector and plasmid DNA testing services market owing to increasing partnership between the key market players for research and development activities. For instance, in April 2020, INOVIO pharmaceuticals Inc., a biotechnology company, entered into an agreement to expand its manufacturing partnership with the Germany-based contract manufacturer Richter-Helm BioLogics GmbH & Co. KG, to support large-scale manufacturing of INOVIO's investigational DNA vaccine INO-4800, which currently is in Phase 1 clinical testing in the U.S. for COVID-19 and could potentially advance to Phase 2/3 efficacy trials this summer.
Viral Vector and Plasmid DNA Testing Services Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 370.4 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 25.8% | 2032 Value Projection: | USD 1,846.9 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Charles River Laboratories, Inc., WuXi AppTec Co., Ltd., Cobra Biologics and Pharmaceutical Services, Merck KgaA, Lonza, Eurofins Scientific, FinVector Vision Therapies, Advanced Bioscience Laboratories, Inc., Takara Bio Inc., ViruSure GmbH, Genezen Laboratories, Akron Biotech., Catalent, Inc, AcuraBio., CATUG Biotechnology., Creative Biogene and Aldevroz |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Viral Vector and Plasmid DNA Testing Services Market- Segmentation
Global viral vector and plasmid DNA testing services market report is segmented into by service type, by end user and by region.
Based on Service Type, the market is segmented into safety, genetic characterization, purity, identity and potency. Out of which, safety segment is expected to hold a dominant position in the global viral vector and plasmid DNA testing services market during the forecast period, owing to increasing number of inorganic growth strategies such as collaboration and others
Based on End User, the market is segmented into pharmaceutical and biotechnology industries and research organizations. Out of which, pharmaceutical and biotechnology industries segment is expected to dominate the market over the forecast period, owing to increasing research and development activities using viral vector and plasmid DNA for various therapeutic applications such as oncology and others
Based on Region, global viral vector and plasmid DNA testing services market is segmented into North America, Latin America, Europe, Middle East, Asia Pacific, and Africa. Out of which, North America is expected to dominate the market over the forecast period, due to increasing inorganic growth strategies such as agreement , partnership and others by key players in the region.
Among all segmentation, pharmaceutical and biotechnology industries segment has the highest potential key players are focusing on adoption of inorganic growth strategies such as partnerships, and collaborations, in order to strengthen their market presence in the global viral vector and plasmid DNA testing services market. For instance, in June 2021, AGC Biologics, a contract development and manufacturing organization (CDMO), announced that it had partnered with BioNTech, a Germany-based biotechnology company, to further supply plasmid DNA (pDNA) starting material for the Pfizer-BioNTech COVID-19 vaccine at AGC’s Heidelberg, Germany facility. Plasmid DNA (pDNA) starting material is an essential component of BioNTech’s mRNA-based vaccine manufacturing process.
Global Viral Vector and Plasmid DNA Testing Services Market- Cross Sectional Analysis
Among service type, safety segment held a dominant position in North America region over the forecast period due to increasing number of inorganic growth strategies such as collaboration and others by the market players for product development. For instance, in In May 2020, AGC Biologics., AGC Biologics, a contract development manufacturing company, and Takara Bio, a biotechnology company, collaborated in order to develop a prophylaxis COVID-19 vaccine candidate. Under this agreement, AGC Biologics will manufacture the plasmid DNA intermediate for the vaccine.
Global Viral Vector and Plasmid DNA Testing Services Market- Key Developments
In January 2022, Sarepta Therapeutics, Inc., a biotechnology company, and Aldevron, A corporate company, collaborated for the supply of GMP grade plasmid to meet the needs of Sarepta’s gene therapy clinical trials and commercial supply.
In August 26, 2021, INOVIO, a biotechnology has announced that it has received regulatory authorization from Brazil's ANVISA (Agência Nacional de Vigilância Sanitária), the national health regulatory agency of Brazil, to initiate the global Phase 3 segment of its Phase 2/3 trial, INNOVATE (INOVIO INO-4800 Vaccine Trial for Efficacy), for INO-4800, its DNA vaccine candidate for COVID-19. INOVIO plans to conduct the global INNOVATE Phase 3 segment in multiple countries, including Brazil, with partner Advaccine Biopharmaceuticals Suzhou Co., Ltd. (Advaccine).
In July 2021, Thermo Fisher Scientific, a pharmaceutical company, announced the opening of its new cGMP (Current good manufacturing practices) plasmid DNA manufacturing facility in Carlsbad California, U.S.
In June, 2020, Genprex, Inc., a clinical-stage gene therapy company, announced collaboration with Aldevron, a biotechnology company, in order to manufacture GMP plasmid DNA for Genprex's Oncoprex gene therapy portfolio.
Global Viral Vector and Plasmid DNA Testing Services Market- Key Trends
Increasing number of investment by market players for product development
Increasing number of investment by the market players in for product development is expected to foster the market growth over the forecast period. For instance, in September, 2022, Trans-Atlantic private equity healthcare specialist, ArchiMed, invested in Bielefeld, Germany-based PlasmidFactory. PlasmidFactory is the contract manufacturer and service provider for plasmid and minicircle DNA. PlasmidFactory develops and manufactures exceptionally high-grade plasmids and minicircle, used to modify cells and produce viral gene therapy vectors such as Adeno-associated virus (AAV), Lentiviral vectors (LV) and mRNA for combating everything from viruses such as COVID-19 to seemingly intractable diseases such as cancer, cystic fibrosis, heart disease, diabetes, hemophilia and AIDs (including CAR-T cell applications). Plasmids are notably a key component for the production of mRNA COVID-19 vaccines.
Partnership by key market players
Key players are focused on partnerships to strengthen their position, is expected to fuel the global viral vector and plasmid DNA testing services market over the forecast period. For instance, on October 17, 2022, GenScript ProBio, a global CDMO, and GeneCraft, a global research of lung cancer mechanism research, announced that they had entered into a strategic partnership MOU concerning the development and production of a new drugs needed for RX001. GenScript ProBio and GeneCraft have agreed to strengthen their cooperation in the AAV gene therapy field through this MoU. GenScript ProBio and GeneCraft are in the process of signing a contract for plasmid and AAV development and production for the GeneCraft's own new drug candidate Pan-KRAS non-small cell lung cancer anti-cancer gene therapy (RX001) developed as a new drug.
Global Viral Vector and Plasmid DNA Testing Services Market: Restraints
Requirement of highly Skilled Labor
Production of recombinant plasmid DNA requires advanced manufacturing equipment and facilities and highly experienced and skilled labor. Limited number of Contract Development and Manufacturing Organizations (CDMOs) and biopharmaceutical companies have enough resources at all stages of the product development cycle. Majority of CDMOs providing commercial and clinical manufacturing and development services and academic laboratories providing early phase support, are running at high capacity, typically with longer wait list.
Challenges Faced During DNA Supply
Biotech and pharmaceutical companies that are majorly focused on advanced therapy manufacturing face challenges associated with their plasmid DNA supply, which includes slow or delayed production and poor DNA quality, and impacting their ability to meet timelines and regulatory demands. However, the regenerative medicine industry is also experiencing challenges and limitations with genetic medicines. Almost, every genetic medicines today are dependent on plasmid DNA as either critical starting material or API (Application Programming Interface), which presents challenges in advanced therapy manufacturing.
Global Viral Vector and Plasmid DNA Testing Services Market- Key Players
Major players operating in the global viral vector and plasmid DNA testing services market include Charles River Laboratories, Inc., WuXi AppTec Co., Ltd., Cobra Biologics and Pharmaceutical Services, Merck KgaA, Lonza, Eurofins Scientific, FinVector Vision Therapies, Advanced Bioscience Laboratories, Inc., Takara Bio Inc., ViruSure GmbH, Genezen Laboratories, Akron Biotech., Catalent, Inc, AcuraBio., CATUG Biotechnology., Creative Biogene and Aldevroz
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients