Global veterinary oncology market is estimated to be valued at USD 810.5 Mn in 2025 and is expected to reach USD 1,770.6 Mn by 2032, exhibiting a compound annual growth rate (CAGR) of 11.8% from 2025 to 2032.
To learn more about this report, Download Free Sample
The global veterinary oncology market is expanding steadily, driven by rising pet ownership, increased awareness of animal health, and advancements in cancer diagnostics and treatments. Companion animals dominate the market due to growing emotional attachment and spending on pet care. Canine lymphoma leads among cancer types treated. Hospital pharmacies are key distribution hubs, offering specialized care.
|
Current Event |
Description and its impact |
|
Rise in Pet Cancer Cases Globally |
|
|
Increased Pet Insurance Adoption |
|
|
Expansion of Veterinary Specialty Clinics |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
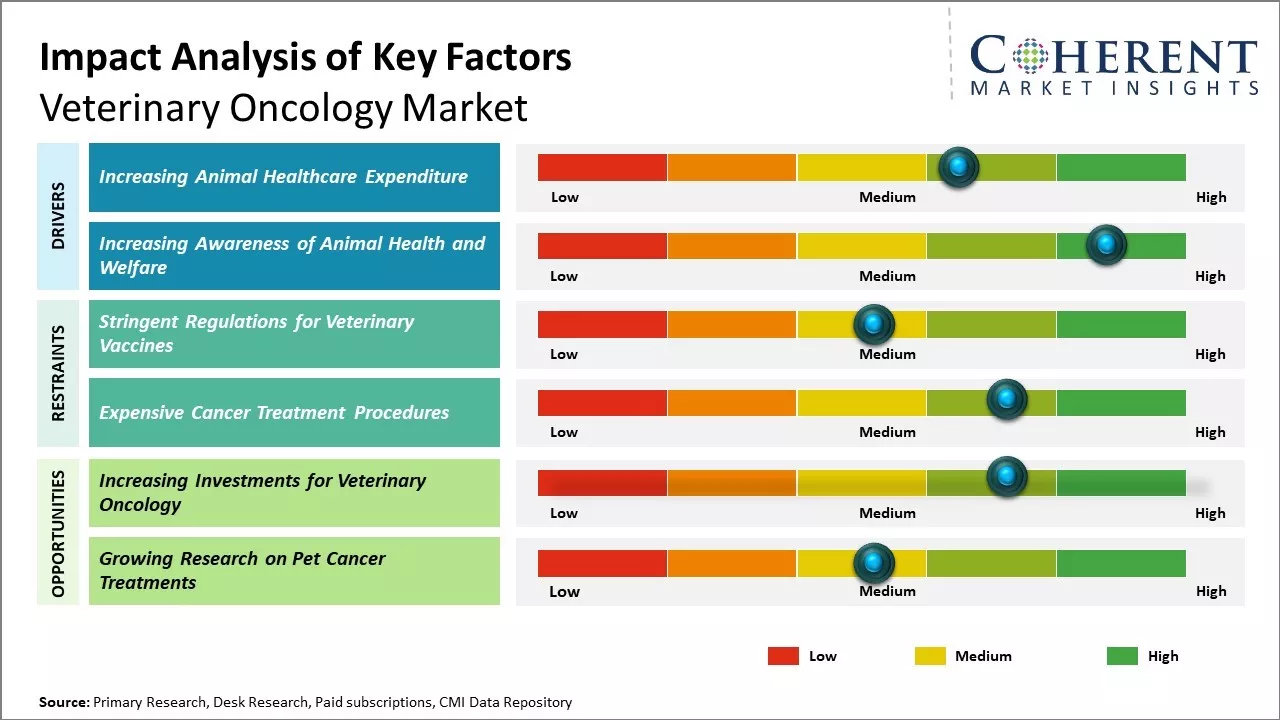
Increasing animal healthcare expenditure is expected to drive the global veterinary oncology market Iover the forecast period. For instance, in May 2023, the North American Pet Health Insurance Association (NAPHIA) released its 2023 State of the Industry (SOI) report showing that the North American pet health insurance sector grew by 23.5% in In-Force GWP over 2022.
Increasing awareness of animal health and welfare and shifts in demographics and lifestyles leading to increased spending on pet healthcare are expected to drive the market growth over the forecast period. For instance, in May 2021, - Petco Health and Wellness Company, Inc., a complete partner in pet health and wellness, launched Together Strong, a pet cancer awareness campaign that brings two- and four-legged survivors together to highlight how routine preventative care is as vital for pets as it is for humans in the fight against cancer.
Along with the campaign and in partnership with PetDx, a molecular diagnostics company aiming to revolutionize the detection, characterization and management of cancer in pets, Petco will begin offering OncoK9 a ground-breaking multi-cancer early detection test for dogs – at its full-service vet hospitals in select locations.
Stringent regulations for veterinary vaccines across various geographies are proving to be a major restraining factor for the growth of the global veterinary oncology market. Regulatory authorities across countries have implemented strict norms and guidelines for the development, manufacturing and approval of new veterinary vaccines to ensure safety, efficacy, and quality.
Complying with these complex regulations involves extensive clinical trials, comprehensive documentation and significant investments of both time and funds. This lengthens the overall product development lifecycle considerably and delays the market introduction of new oncology vaccines.
Increasing investments for veterinary oncology healthcare is expected to offer lucrative opportunities in the market. For instance, in December 2021, PetDx, the start up behind an early cancer-detecting blood test for dogs, raised the funding of USD 62 million to get canine cancer-detecting tech to vets across the U.S. OncoK9 can detect 30 cancers from a blood draw in front of more veterinarians and become a standard test in pet clinics across the country.
The companion animal segment is estimated to dominate the veterinary oncology market with a 59.8% share in 2025. This growth is primarily driven by the rising trend of pet ownership worldwide, especially in urban areas. Dogs and cats are increasingly treated as family members, leading to heightened awareness and demand for advanced veterinary care.
As cancer becomes one of the leading causes of death among pets, the demand for timely and effective oncology treatment options continues to rise sharply in this segment.
The canine lymphoma segment is projected to hold 32.2% of the veterinary oncology market in 2025. The large global population of dogs, combined with the high incidence of lymphoma, is driving demand for accurate diagnostics and effective treatments. This segment’s growth is also supported by advancements in chemotherapy and immunotherapy protocols, along with increasing veterinary awareness and availability of specialized cancer care for dogs.
The hospital pharmacies segment is expected to dominate the distribution channel category, holding 40.7% of the market share in 2025. Veterinary hospitals play a critical role in dispensing specialized oncology medications and offering integrated cancer care services. Their access to advanced facilities and trained professionals allows for more comprehensive treatment options, including chemotherapy and radiation, which supports this segment’s leading position in the market.
To learn more about this report, Download Free Sample
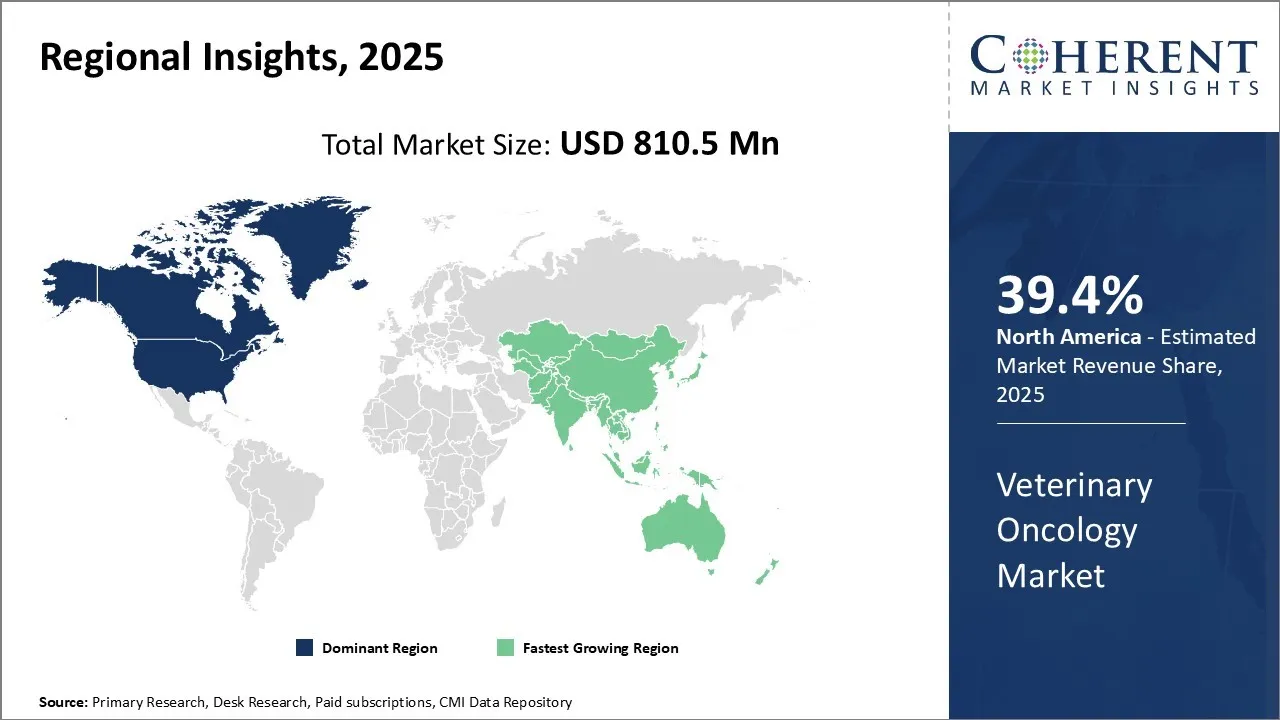
North America is anticipated to maintain its dominance in the global veterinary oncology market with a 39.4% share in 2025. This leadership is driven by strong economic conditions, high pet ownership rates, and greater awareness regarding pet healthcare. In the U.S. and Canada, increasing disposable incomes enable pet owners to invest in premium veterinary services, including advanced cancer diagnostics and treatments. The region also benefits from a well-established veterinary infrastructure, widespread availability of oncology specialists, and robust insurance coverage for pets.
Continuous innovation by pharmaceutical companies and the presence of veterinary teaching hospitals further strengthens North America’s position as the global leader in animal cancer care. Additionally, growing collaborations between research institutions and veterinary clinics support the development and accessibility of cutting-edge therapies such as immunotherapy and precision medicine.
Europe is expected to witness strong growth in the veterinary oncology market over the forecast period, supported by rising pet adoption and increasing prioritization of animal welfare. Countries such as Germany, France, and the UK are seeing a surge in pet healthcare expenditure, which includes early cancer detection and treatment services. The region's strict regulatory framework and strong veterinary education systems have resulted in a high standard of oncology care across both urban and rural settings.
Additionally, European governments and NGOs are actively promoting preventive veterinary healthcare and funding research into animal diseases, including cancer. The growing presence of pet insurance, coupled with expanding veterinary clinic networks, is making oncology treatments more accessible. With its commitment to quality care and innovation, Europe is emerging as a key hub for veterinary oncology development and adoption.
The United States and Canada are the leading countries in the global veterinary oncology market, with North America projected to account for 39.4% of the market share in 2025. The U.S. boasts a highly developed veterinary healthcare infrastructure, widespread pet insurance coverage, and a growing focus on companion animal wellness, all of which contribute to high adoption rates of advanced cancer diagnostics and therapies.
Canada complements this with increasing pet ownership, expanding veterinary service networks, and government support for animal health research. Together, these countries dominate the regional market through clinical advancements, research collaborations, and the availability of specialized veterinary oncology services.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 810.5 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 11.8% | 2032 Value Projection: | USD 1,770.6 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Zoetis Inc., Elanco, AB Science, Boehringer Ingelheim International GmbH, Zenoaq, VetDC, Morphogenesis, Inc., Karyopharm Therapeutics, Inc., Regeneus Ltd., AdvaVet, Inc., Rhizen Pharmaceutical SA., PetCure Oncology, Varian Medical Systems, Accuray Incorporated, Merck Animal Health, Regeneus Ltd., and VolitionRx Limited |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients