Venous Thromboembolism Market Size and Forecast – 2026 – 2033
The global venous thromboembolism market is estimated to be valued at USD 5.8 billion in 2026 and is projected to reach USD 9.6 billion by 2033, growing at a compound annual growth rate (CAGR) of 7.2% from 2026 to 2033.
Global Venous Thromboembolism Market Overview
The venous thromboembolism (VTE) market products include anticoagulant drugs, thrombolytic agents, mechanical devices, and diagnostic solutions. Thrombolytic drugs are used in severe cases to rapidly dissolve clots. Mechanical products, including compression stockings, intermittent pneumatic compression devices, and inferior vena cava (IVC) filters, support prevention and management. Diagnostic products such as D-dimer tests and imaging systems enable early detection, improving treatment outcomes and reducing VTE-related complications.
Key Takeaways
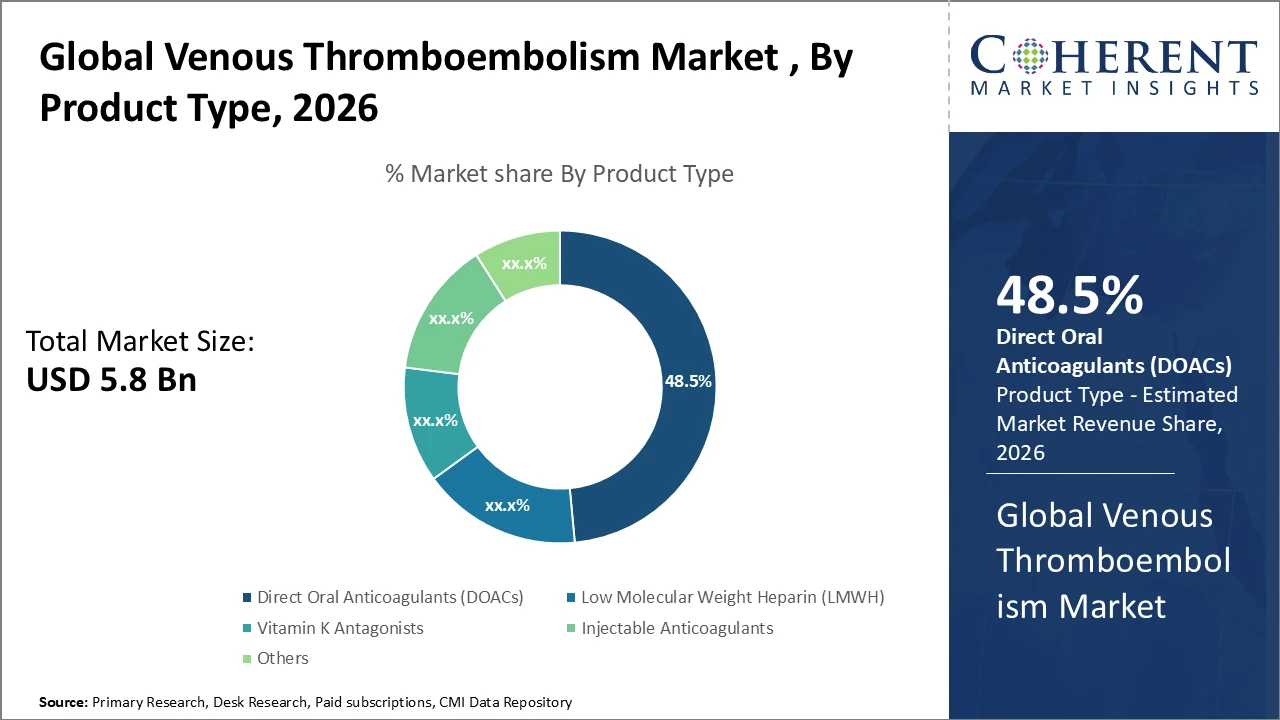
• The direct oral anticoagulants (DOACs) segment leads the market with a 48.5% share, driven by broad clinical adoption and favorable reimbursement frameworks.
• Hospitals account for 60.3% of the total market share, supported by advanced infrastructure, higher patient inflow, and greater procedural volumes.
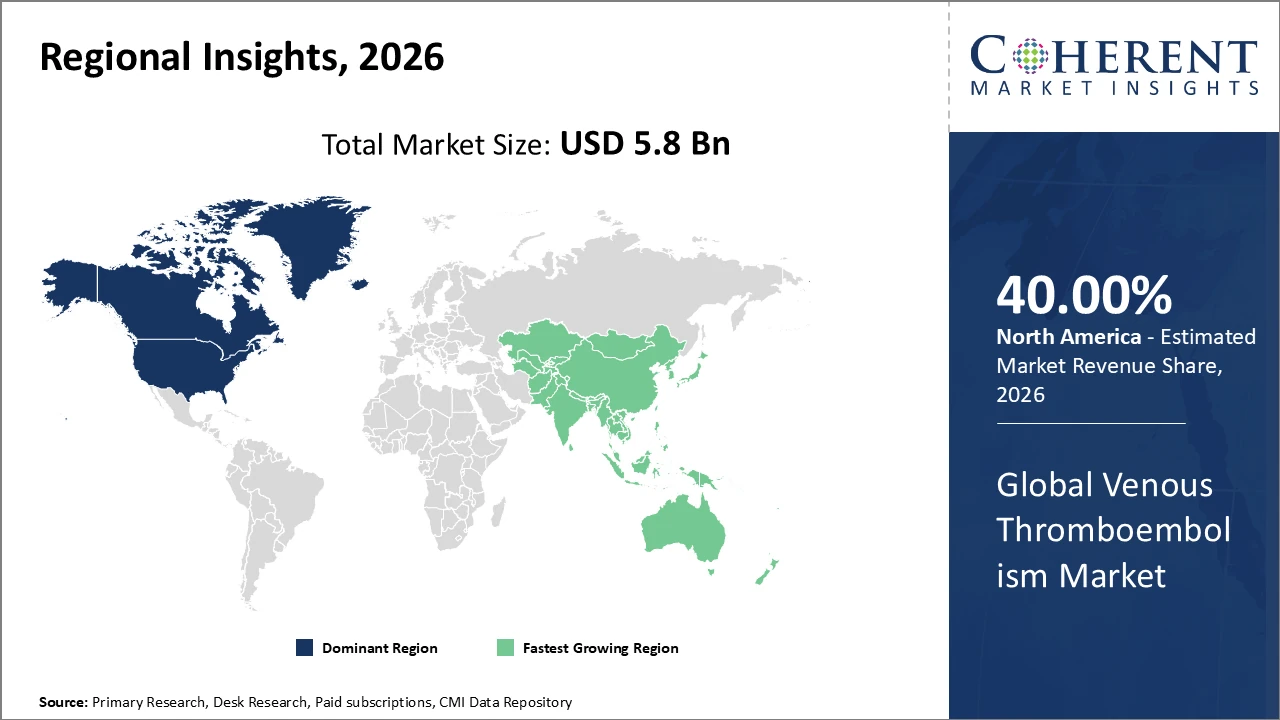
• North America holds the largest share of venous thromboembolism market revenue due to strong healthcare systems, early drug approvals, and high awareness levels.
• Asia Pacific is expected to register the fastest CAGR, fueled by rising healthcare investments, improving access to treatment, and a growing aging population at risk of VTE.
Venous Thromboembolism Market Segmentation Analysis
To learn more about this report, Download Free Sample
Venous Thromboembolism Market Insights, By Product Type
Direct oral anticoagulants (DOACs) dominate the market due to their convenient oral administration, lower risk of bleeding complications, and growing endorsement in clinical guidelines. Recent data indicate that global DOAC prescriptions increased by 22% between 2024 and 2026, driven largely by strong adoption of apixaban and rivaroxaban across Europe and North America. Low-molecular-weight heparin (LMWH) continues to play a vital role, particularly in inpatient care and prophylactic use, accounting for a significant share of hospital anticoagulant consumption. Vitamin K antagonists (VKAs), while historically important, are steadily declining because of complex monitoring requirements. Injectable anticoagulants remain standard in post-surgical care, maintaining stable market share. The “others” segment, comprising biosimilars and novel anticoagulants in clinical development, represents a key innovation area with potential to reshape future market dynamics.
Venous Thromboembolism Market Insights, By Application
Prophylaxis holds a dominant market share, driven by the increasing emphasis on preventive care for high-risk surgical and hospitalized patients. In 2025, global use of prophylactic anticoagulants grew at an estimated annual rate of 15%, with particularly strong uptake in orthopedic and oncologic care settings. The treatment segment is expanding rapidly, supported by improved diagnostic capabilities and a rising incidence of acute VTE events. Post-thrombotic syndrome management remains a niche area but is expected to gain traction as awareness of long-term VTE complications increases and more effective management protocols are adopted. The “others” category includes experimental and off-label applications, which currently have limited adoption but present potential future growth opportunities as clinical evidence evolves.
Venous Thromboembolism Market Insights, By End-User
Hospitals hold the largest share of the venous thromboembolism market, driven by high patient volumes and advanced treatment capabilities, accounting for over 60% of total revenue. They serve as primary centers for acute VTE management and postoperative prophylaxis. Ambulatory surgical centers represent the fastest-growing end-user segment, supported by the rising number of outpatient surgeries and increased adoption of minimally invasive procedures that enable efficient anticoagulant administration and monitoring. Clinics and home healthcare settings are emerging due to advances in telemedicine and growing patient preference for outpatient care, though they currently account for a smaller share. The “others” segment includes rehabilitation centers and specialty care units, contributing modestly to overall market revenue.
Venous Thromboembolism Market Trends
• The venous thromboembolism market is shifting toward patient-centric care models, supported by improvements in diagnostic accuracy and therapeutic precision.
• Adoption of direct oral anticoagulants (DOACs) continues to rise due to strong clinical outcomes and better patient adherence, with global prescriptions increasing by around 20% during 2024–2026.
• AI-powered predictive and risk-stratification tools are increasingly used to personalize anticoagulation therapy and improve clinical decision-making.
• Regulatory agencies in major markets are accelerating approvals for novel anticoagulant agents, improving market access and encouraging innovation.
• Biosimilar penetration is increasing, particularly in Asia Pacific and Latin America, enhancing treatment affordability and expanding market reach.
• Biosimilar products accounted for more than 15% of anticoagulant market revenues in 2025, significantly influencing competitive dynamics and pricing strategies.
Venous Thromboembolism Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Venous Thromboembolism Market Analysis and Trends
North America holds a dominant position with a share of 40%, driven by well-established healthcare infrastructure, high research and development spending, and the strong presence of leading pharmaceutical companies. The United States accounts for the largest share within the region, supported by widespread adoption of advanced therapeutic options and favorable reimbursement policies. Continuous innovation in anticoagulant drugs and diagnostic technologies has further strengthened market growth, enabling early detection and effective management of VTE. These factors collectively reinforce North America’s leading position in the global venous thromboembolism market.
Asia Pacific Venous Thromboembolism Market Analysis and Trends
Asia Pacific is expected to register the fastest growth in the venous thromboembolism market, supported by rising healthcare investments, expanding elderly populations, and increasing awareness of thrombotic disorders. Countries such as China and India are experiencing rapid improvements in healthcare infrastructure alongside regulatory reforms that enable faster drug approvals. The region’s growth is further driven by rising demand for cost-effective anticoagulant therapies and broader access to treatment. As a result, Asia Pacific’s CAGR is projected to outpace that of other regions, positioning it as a key growth engine for the global market.
Venous Thromboembolism Market Outlook for Key Countries
USA Venous Thromboembolism Market Analysis and Trends
The U.S. venous thromboembolism market serves as a benchmark for global industry trends, propelled by innovations in anticoagulant therapies and advanced diagnostics. The country benefits from a mature healthcare system and supportive government policies that facilitate the development and rapid uptake of novel therapies. Additionally, strategic collaborations among major pharmaceutical companies have streamlined R&D processes, accelerated the commercialization of next-generation anticoagulants and reinforcing the U.S. market’s leadership in VTE management.
Germany Venous Thromboembolism Market Analysis and Trends
Germany’s venous thromboembolism (VTE) market is characterized by steady growth, driven by advanced healthcare infrastructure, high-quality diagnostic facilities, and early adoption of novel anticoagulant therapies. Emerging biosimilars and ongoing clinical trials offer additional growth opportunities, shaping competitive dynamics and innovation within the German VTE landscape.
Analyst Opinion
• A key supply-side driver of the venous thromboembolism market is the expansion of production capacities by pharmaceutical companies, particularly for direct oral anticoagulants (DOACs). By the end of 2025, global DOAC production increased by nearly 18% to meet rising demand in outpatient treatment settings, positively impacting market revenue, especially in regions with growing outpatient healthcare infrastructure.
• On the demand side, hospital admissions due to post-surgical thrombosis complications have risen, with surgical-related VTE incidences increasing by 12% from 2024 to 2026 in major developed markets. This trend has strengthened the demand for prophylactic therapies, boosting the market share of injectable anticoagulants in tertiary care.
• Micro-level dynamics show increased adoption of biomarker-driven diagnostics. The use of D-dimer assays and venous duplex ultrasonography improved early detection rates by 15% between 2024 and 2026, accelerating treatment initiation and expanding the market for diagnostic devices.
• Pricing and reimbursement strategies have significantly influenced market growth. Competitive pricing and favorable reimbursement policies in emerging economies led to a 10% rise in market penetration for generic low-molecular-weight heparin (LMWH) formulations in 2025, highlighting the impact of affordability on market expansion.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 5.8 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 7.2% | 2033 Value Projection: | USD 9.6 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Pfizer Inc., Bayer AG, Johnson & Johnson, Sanofi S.A., Novartis AG, Abbott Laboratories, CSL Limited, Mylan N.V., Grifols, S.A., Cipla Limited | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Venous Thromboembolism Market Growth Factors
The rising prevalence of vascular disorders among aging populations is a key driver of venous thromboembolism market growth, with the World Health Organization reporting a 20% increase in VTE cases among individuals over 65 between 2024 and 2026. Improved screening and diagnostic capabilities further boost market revenue, as timely intervention with advanced anticoagulants reduces mortality and shortens hospital stays. Technological advancements in point-of-care testing devices have accelerated adoption by healthcare providers, expanding market reach in ambulatory and home-care settings. Favorable regulatory frameworks supporting fast-tracked approvals, such as the expedited FDA clearance of next-generation factor Xa inhibitors in early 2026, have further stimulated market growth.
Venous Thromboembolism Market Development
In October 2025, Cardinal Health announced the global launch of the Kendall SCD SmartFlow™ Compression System, the latest advancement in the Kendall™ Compression Series. The system uses clinically validated technology to deliver customised intermittent pneumatic compression (IPC), improving blood flow in patients at risk. It is designed to help prevent venous thromboembolism (VTE), enhance circulation, and reduce pain and swelling associated with venous stasis, while also improving both clinician usability and patient comfort.
Key Players
Leading Companies of the Market
Pfizer Inc.
Bayer AG
Johnson & Johnson
Sanofi S.A.
Novartis AG
Abbott Laboratories
CSL Limited
Mylan N.V.
Grifols, S.A.
Cipla Limited
Several companies in the venous thromboembolism market have strengthened strategic collaborations and acquisitions to broaden their product offerings and geographic reach. For instance, Pfizer’s collaboration with BioNTech in 2025 focused on optimizing anticoagulant delivery, achieving a 25% improvement in bioavailability in clinical trials. Similarly, Bayer’s 2024 acquisition of a leading biosimilar anticoagulant manufacturer boosted its presence in emerging markets, resulting in a 14% year-over-year revenue increase in those regions. These initiatives highlight how partnerships and acquisitions are driving innovation, market expansion, and competitive advantage in the VTE landscape.
Venous Thromboembolism Market Future Outlook
The venous thromboembolism (VTE) market is poised for strong growth, driven by rising prevalence of thrombotic disorders, aging populations, and increasing awareness of preventive care. Expansion of direct oral anticoagulants (DOACs), coupled with advancements in biomarker-driven diagnostics and AI-powered risk assessment tools, will enhance early detection and personalized treatment. Emerging biosimilars and novel anticoagulants in clinical development are expected to improve affordability and diversify therapeutic options. Additionally, growing adoption of outpatient and home-care models, along with supportive regulatory frameworks, will further expand market reach. Overall, innovation, accessibility, and patient-centric care will define the market’s trajectory in the coming decade.
Venous Thromboembolism Market Historical Analysis
The venous thromboembolism (VTE) market has experienced steady growth over the past decade, primarily driven by increasing incidence of deep vein thrombosis and pulmonary embolism, especially among aging populations. Historically, vitamin K antagonists (VKAs) and low-molecular-weight heparin (LMWH) dominated therapy, with hospitals serving as the primary end-users. Growing awareness of VTE risks, improvements in diagnostic techniques such as D-dimer assays and venous ultrasonography, and the introduction of direct oral anticoagulants (DOACs) from the early 2010s reshaped treatment paradigms. Expansion of prophylactic strategies in surgical and high-risk patients further boosted market adoption, setting the stage for rapid innovation in subsequent years.
Sources
Primary Research Interviews:
Hematologists and Cardiologists
Vascular Surgeons
Hospital Pharmacists
Clinical Trial Investigators
Databases:
World Health Organization (WHO) Health Statistics
OECD Health Data
Centers for Disease Control and Prevention (CDC)
Magazines:
Medscape Medical News
PharmaTimes
Cardiovascular Business
HealthTech Magazine
Medical Device Network
Journals:
Journal of Thrombosis and Haemostasis
Blood Advances
American Journal of Hematology
Vascular Medicine
Thrombosis Research
Newspapers:
The New York Times (Health)
The Guardian (Health)
Financial Times (Healthcare)
The Hindu (Health)
Reuters Health
Associations:
World Health Organization (WHO)
International Society on Thrombosis and Haemostasis (ISTH)
American Heart Association (AHA)
National Blood Clot Alliance (NBCA)
European Society of Cardiology (ESC)
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients