Vasculitis Treatment Market Size and Forecast – 2026 – 2033
The Vasculitis Treatment Market is projected to grow from about USD 3.5 billion in 2026 to around USD 5.2 billion by 2033, expanding at an approximate CAGR of 5.5 % from 2026–2033. Growth is driven by rising disease prevalence, novel therapies, and advanced biologics adoption.
Global Vasculitis Treatment Market Overview
Vasculitis treatment focuses on reducing blood vessel inflammation, controlling symptoms, and preventing organ damage. Therapy depends on disease type, severity, and organs involved. Common treatments include corticosteroids for rapid inflammation control, immunosuppressive drugs such as cyclophosphamide and methotrexate, and biologic agents like rituximab for targeted immune modulation. Mild cases may require only short-term medication, while severe or chronic vasculitis often needs long-term therapy and monitoring. Advances in biologics and personalized medicine have improved outcomes, reduced relapse rates, and minimized treatment-related side effects. Early diagnosis and tailored treatment are critical for improving patient prognosis.
Key Takeaways
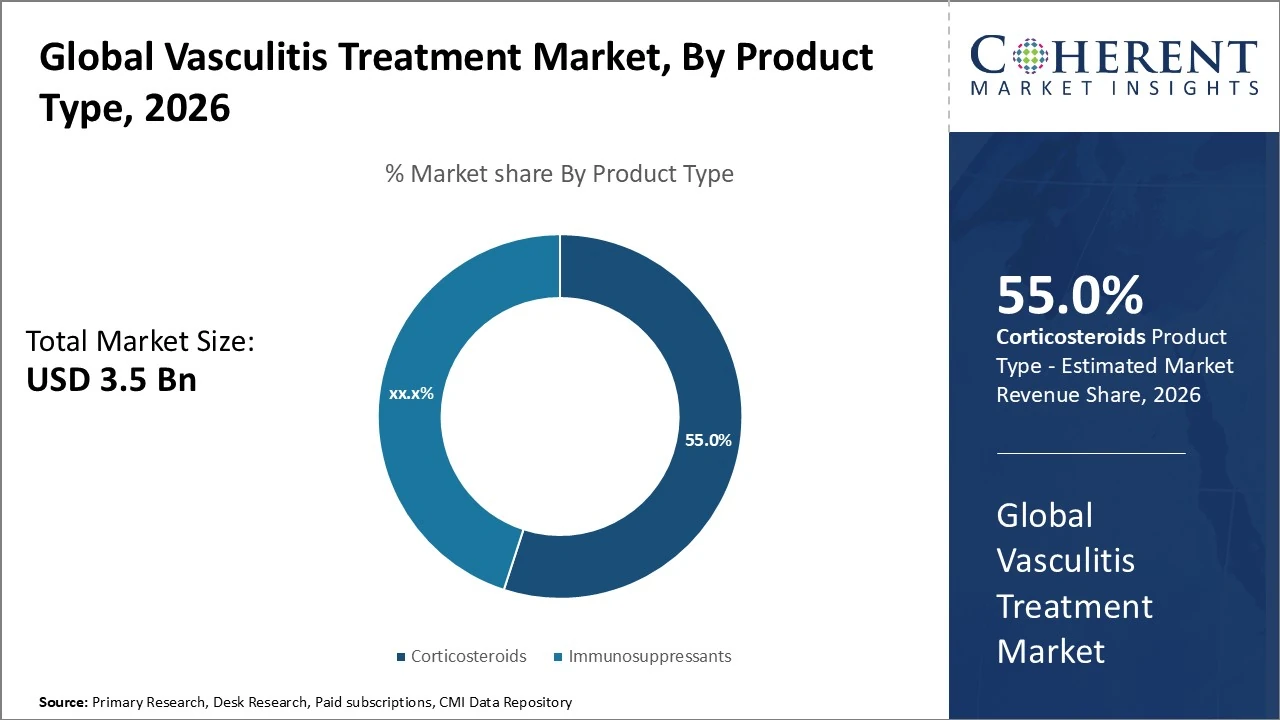
By Product Type- Corticosteroids remain dominant, representing the largest share which is 55% of treatments.
In the vasculitis treatment market, hospitals are the dominant end-user, accounting for approximately 48% of market share.
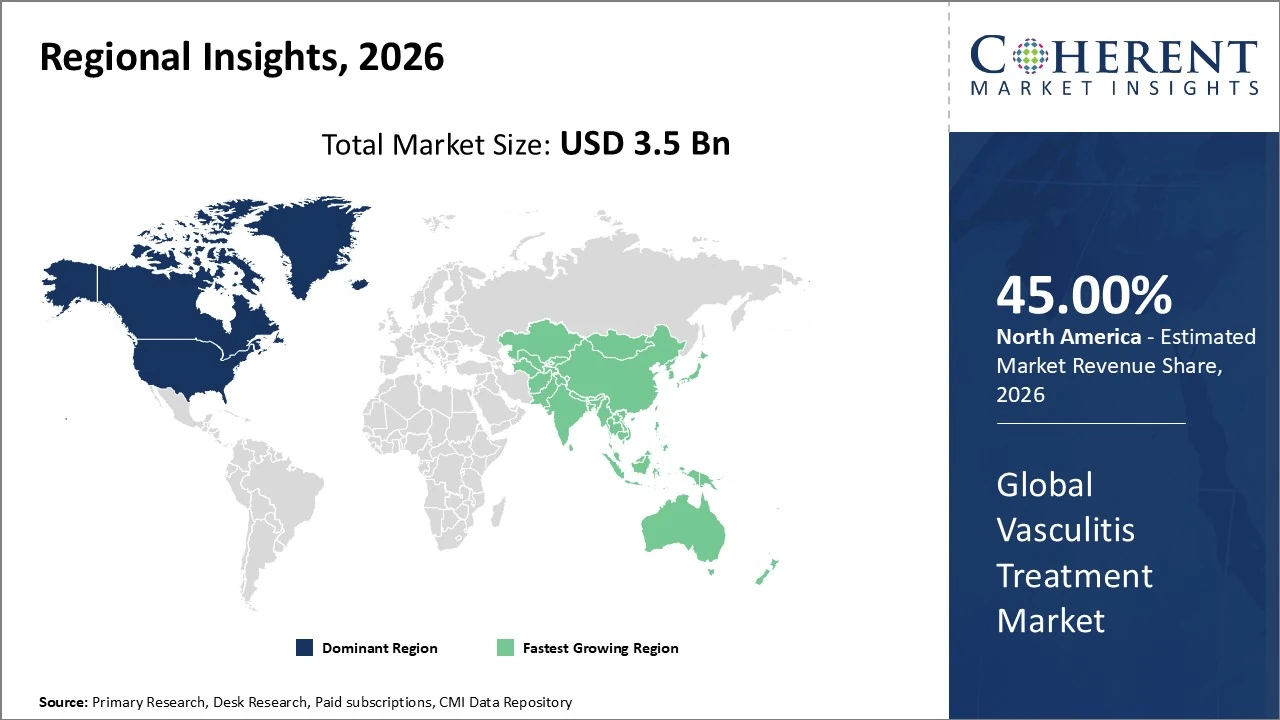
North America dominates the Vasculitis Treatment Market, holding about 45% of global revenue share.
The Asia Pacific vasculitis treatment market currently accounts for roughly 18% of the global vasculitis market.
Vasculitis Treatment Market Segmentation Analysis
To learn more about this report, Download Free Sample
Vasculitis Treatment Market Insights, By Product Type
Corticosteroids remain dominant, representing the largest share which is 55% of treatments due to their long-standing use in inflammation control. Immunosuppressants also hold a significant portion, frequently around 45% in specialized vasculitis subsets, driven by broad clinical adoption and cost-effectiveness. Market growth reflects shifting preferences toward precision biologic solutions alongside traditional therapies.
Vasculitis Treatment Market Insights, By Technology
In the vasculitis treatment market, immunosuppressive therapies still hold a significant share, accounting for about 60 % of technology usage due to established clinical adoption and cost-effectiveness. Advanced biologic therapies targeting specific immune pathways represent the fastest-growing technology segment, capturing roughly 40 % of prescriptions as clinicians shift toward precision immunomodulation. Biologics—including interleukin inhibitors and B-cell depleting agents—offer targeted action with fewer systemic effects, driving adoption. Precision medicine and biomarker-based approaches further expand biologics’ role, improving efficacy and reducing side effects compared with conventional treatment methods.
Vasculitis Treatment Market Insights, By End-User
In the vasculitis treatment market, hospitals are the dominant end-user, accounting for approximately 48% of market share due to their comprehensive diagnostic facilities, multidisciplinary teams, and capacity for acute care and biologic infusions. Specialty clinics and outpatient medical clinics represent the next significant segment at roughly 27%, driven by growth in decentralized and personalized care models. Diagnostic centers and homecare/others collectively make up about 25%, supported by rising telemedicine use and home infusion therapies that improve convenience and adherence. Clinics are the fastest-growing end-user as patient preference shifts toward accessible, localized treatment.
Vasculitis Treatment Market Trends
Increasing adoption of biologic therapies and personalized treatment approaches improves efficacy and reduces side effects compared with traditional broad-spectrum drugs.
Telemedicine, remote monitoring tools, and advanced diagnostics (e.g., biomarkers, imaging) are enhancing patient management and early diagnosis.
Expanding clinical trials and development of new agents, including biosimilars and drugs for rare vasculitis forms, boost treatment options and address unmet needs.
Vasculitis Treatment Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Vasculitis Treatment Market Analysis and Trends
North America dominates the Vasculitis Treatment Market, holding about 45% of global revenue share due to advanced healthcare infrastructure, high R&D investment, and early adoption of biologics. The U.S. leads within the region, contributing roughly 76% of North America’s vasculitis treatment revenue. Market growth is supported by rising autoimmune disease prevalence and strong clinical trial activity. The North American segment is projected to grow at a ~7.0% CAGR through 2033, driven by biologics, precision medicine trends, and supportive reimbursement policies.
Asia Pacific Vasculitis Treatment Market Analysis and Trends
The Asia Pacific vasculitis treatment market currently accounts for roughly 18% of the global vasculitis market, led by rising autoimmune disease prevalence and strengthening healthcare infrastructure across China, Japan, and India. The regional market is expanding at a projected ~9% CAGR through 2026, outpacing developed regions due to increasing diagnosis rates, greater access to biologics, and growing patient awareness. Rapid urbanization, rising disposable incomes, and expanding specialty care facilities are key growth drivers, though treatment affordability remains a challenge in developing areas.
Vasculitis Treatment Market Outlook for Key Countries
USA Vasculitis Treatment Market Analysis and Trends
The U.S. vasculitis treatment market is the largest within North America, capturing about ~76% of the regional ANCA vasculitis therapy revenue share in 2026, driven by advanced healthcare infrastructure and high adoption of targeted biologic therapies. Biologics account for roughly 38% of prescribed treatments, with corticosteroids still at 62% in standard care. The market is growing at an estimated ~8% CAGR through 2026 due to increased disease awareness, improved diagnostics, and personalized therapy adoption. Expansion of clinical trials and supportive reimbursement policies further propel market growth.
Germany Vasculitis Treatment Market Analysis and Trends
The Germany vasculitis treatment market is a significant part of Europe’s vasculitis landscape, representing roughly ~29% of the European market share and valued at around USD 0.30 billion in 2026. The market is projected to grow at a steady ~6.5% CAGR through 2026, supported by advanced healthcare infrastructure, high autoimmune disease awareness, and broad access to biologic therapies.
Analyst Opinion
Analysts highlight that targeted biologic therapies are increasingly replacing conventional corticosteroids, expected to capture ~37% of market share by 2033 due to better efficacy and fewer long-term side effects.
The U.S. dominates the global market with ~45% revenue share, fueled by advanced healthcare, strong R&D pipelines, and early adoption of innovative therapies.
Asia Pacific shows the fastest growth (~9% CAGR), driven by rising disease awareness, urbanization, and expanding specialty healthcare infrastructure.
Early detection via advanced imaging and biomarker testing is expected to significantly influence market expansion and improve treatment outcomes.
High biologic costs and reimbursement inconsistencies in developing regions may limit adoption, representing a key barrier for market penetration outside major economies.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 3.5 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 5.5% | 2033 Value Projection: | USD 5.2 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | F. Hoffmann‑La Roche Ltd, GlaxoSmithKline plc, AbbVie Inc., Bristol‑Myers Squibb Company, Novartis AG, Pfizer Inc. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Vasculitis Treatment Market Growth Factors
The vasculitis treatment market is expanding due to rising prevalence of autoimmune and inflammatory diseases, especially ANCA-associated vasculitis, coupled with increasing awareness and early diagnosis. Technological advancements in biologics and targeted therapies are improving patient outcomes, driving adoption over traditional corticosteroids. Growth is further supported by expanding healthcare infrastructure, particularly in North America and Asia Pacific, and favorable reimbursement policies in developed regions. Clinical trial activity and drug pipeline innovation enhance market potential.
Key Players
Leading Companies of the Market
F. Hoffmann‑La Roche Ltd
AbbVie Inc.
Novartis AG
Bristol Myers Squibb Company
Pfizer Inc.
Vasculitis Treatment Market Future Outlook
The vasculitis treatment market is poised for steady growth over the next decade, driven by expanding adoption of biologics and targeted therapies and ongoing clinical trial activity. Emerging regions like Asia Pacific are expected to outpace mature markets, benefiting from improved healthcare infrastructure and rising disease awareness. Technological advances in diagnostics, personalized medicine, and biomarker-based therapies will enable earlier detection and more effective treatment. Strategic collaborations, mergers, and pipeline innovations will further strengthen market dynamics. Challenges such as high therapy costs and limited access in developing regions may persist, but overall CAGR is projected at ~5.5% globally.
Vasculitis Treatment Market Historical Analysis
Historically, the vasculitis treatment market was dominated by corticosteroids and conventional immunosuppressants, providing symptomatic relief but with significant long-term side effects. Over the last 10–15 years, the introduction of biologic therapies like rituximab and tocilizumab revolutionized treatment, improving efficacy and reducing relapse rates. North America led early market growth, contributing ~45% of global revenue, followed by Europe (~30%). Limited awareness and healthcare infrastructure constrained growth in Asia Pacific and Latin America. Investment in research, clinical trials, and diagnostic advancements gradually expanded treatment accessibility and patient outcomes, setting the stage for the current and future market expansion.
Sources
Primary Research Interviews:
Healthcare Professionals
Hospital Administrators & Clinics
Pharmaceutical & Biotech Companies
Regulatory Authorities
Databases:
PubMed / Medline
ClinicalTrials.gov
WHO Global Health Observatory
Journals:
Arthritis & Rheumatology
The Journal of Rheumatology
Annals of the Rheumatic Diseases
Clinical Rheumatology
Newspapers:
Financial Times
The Wall Street Journal
The Guardian
The New York Times
Associations:
Vasculitis Foundation (VF)
American College of Rheumatology (ACR)
European Alliance of Associations for Rheumatology (EULAR)
National Organization for Rare Disorders (NORD)
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients