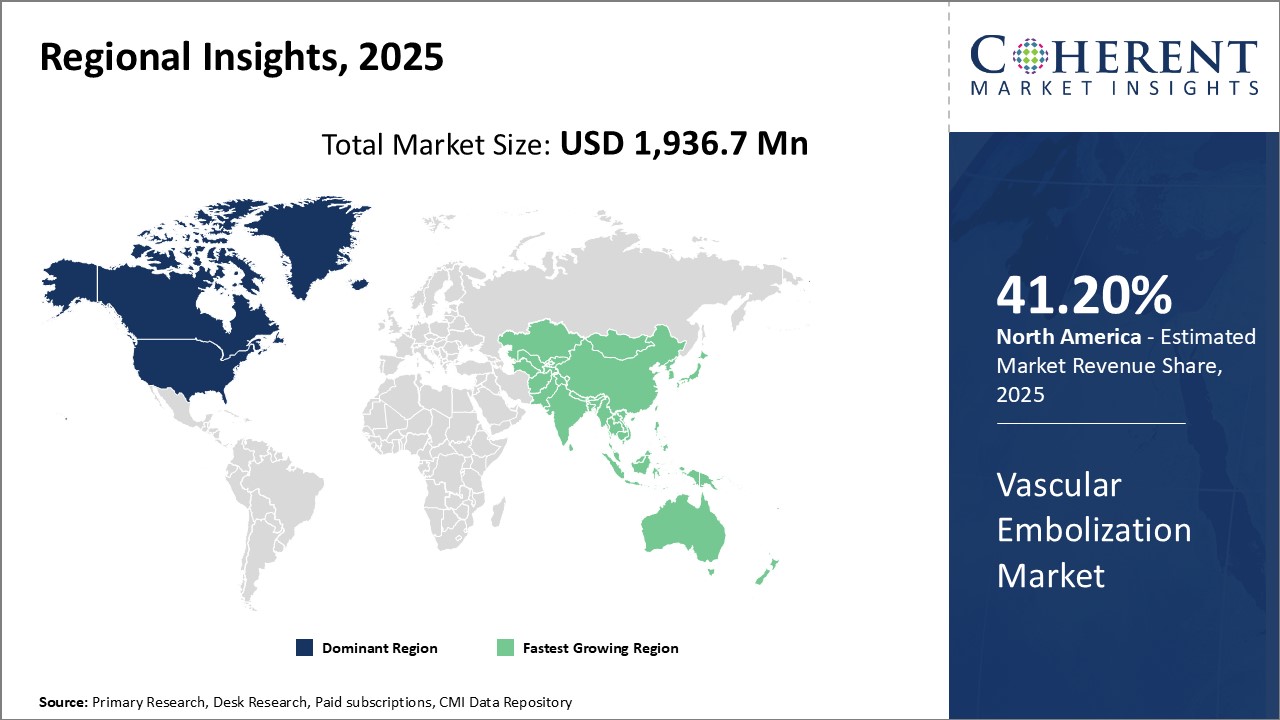
The global vascular embolization market is estimated to be valued at USD 1,936.7 Mn in 2025 and is expected to exhibit a CAGR of 9.7% during the forecast period (2025-2032). Embolization is a minimally invasive treatment (an alternative to open surgery) that blocks one or more blood vessels or abnormal vascular channels. Embolization procedures allow blockage of blood vessels without invasive surgery. Embolization can be used to stop arterial bleeding, and can also be used to block blood vessels for other reasons, such as to treat tumors, re-direct flow, or shrink vascular malformations, among others. Embolization is a very common and effective procedure in interventional radiology. Vascular embolization agents are used to stop flow in blood vessel or vascular area by the means of mechanical occlusion. High success rate, rare use of general anesthetics, faster recovery time, minimal risk of infection, and no scarring are some features of vascular embolization agents.
Market Statistics:
North America held dominant position in the global vascular embolization market in 2020, accounting for 41.2% share in terms of volume, followed by Europe and Asia Pacific, respectively.
Figure 1. Global Vascular Embolization Market Value (USD MN), by Region, 2025
To learn more about this report, Download Free Sample
Vascular Embolization Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1,936.7 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.7% | 2032 Value Projection: | USD 3,702.5 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Shape Memory Medical Inc., Terumo Corp, Penumbra Inc., Abbott Laboratories, Stryker Corporation, Cook Medical, Medtronic PLC, Merit Medical Systems Inc., Johnson and Johnson (Cerenovus), and Boston Scientific Corporation, among others |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Market Drivers:
Figure 2. Global Vascular Embolization Market Share, By Embolization Technique, 2025 To learn more about this report, Download Free Sample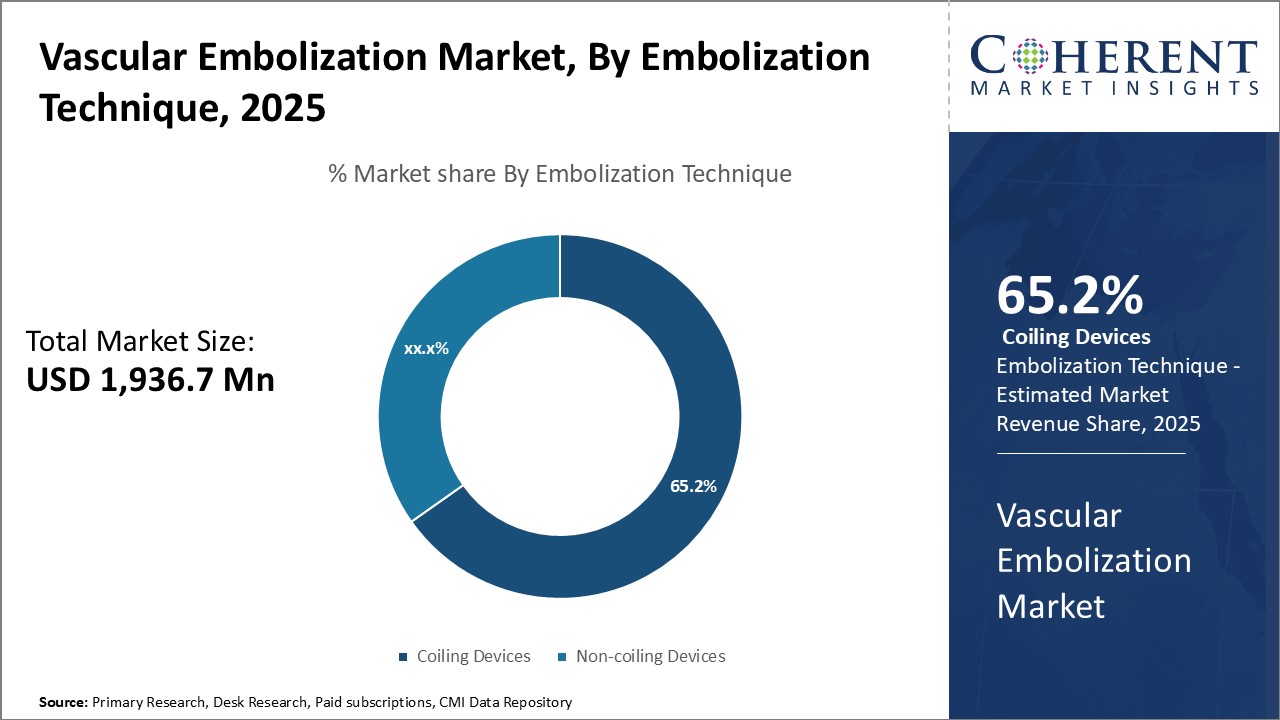
Recent Developments
In January 2023, Fluidx Medical Technology, Inc., a privately-held medical device company, announced the success of the IMPASS Embolic Device in in-vivo studies of middle meningeal artery (MMA) embolizations, which can be used to treat chronic subdural hematomas (CSDH) on the brain's surface.
In November 2022, Varian, a Siemens Healthineers company, announced that the first trial participant had been treated as part of GENESIS II (Genicular Artery Embolization in Patients with Knee Osteoarthritis), a study of embozene microspheres for genicular artery embolization (GAE) as a treatment for mild to moderate knee osteoarthritis. GENESIS II is GAE's largest randomized clinical trial to evaluate pain alleviation for this chronic illness.
In August 2022, Boston Scientific Corporation, a medical device company, announced the acquisition of Obsidio, Inc., a privately-held company that created Gel Embolic Material (GEM) technology for embolizing blood vessels in the peripheral vasculature. GEM technology, approved by the U.S. Food and Drug Administration (FDA), is a semi-solid, patented material packaged in a ready-to-use form, decreasing the preparation time required for various embolization treatments.
In April 2021, Medtronic plc, a global pioneer in medical technology, gained U.S. FDA approval for its Pipeline Flex Embolization Device with Shield Technology. Medtronic plc developed Shield Technology, a proprietary breakthrough in biomaterial science, to advance flow diversion therapy by introducing the first surface-modified implant device with reduced material thrombogenicity, or the ability of the surface treatment material to form clots. NYU Langone Health in New York City completed the U.S’s first patient procedure with the novel technology.
Market Restraints:
Market Opportunities:
Market Trends/Key Takeaways:
Competitive Landscape:
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients