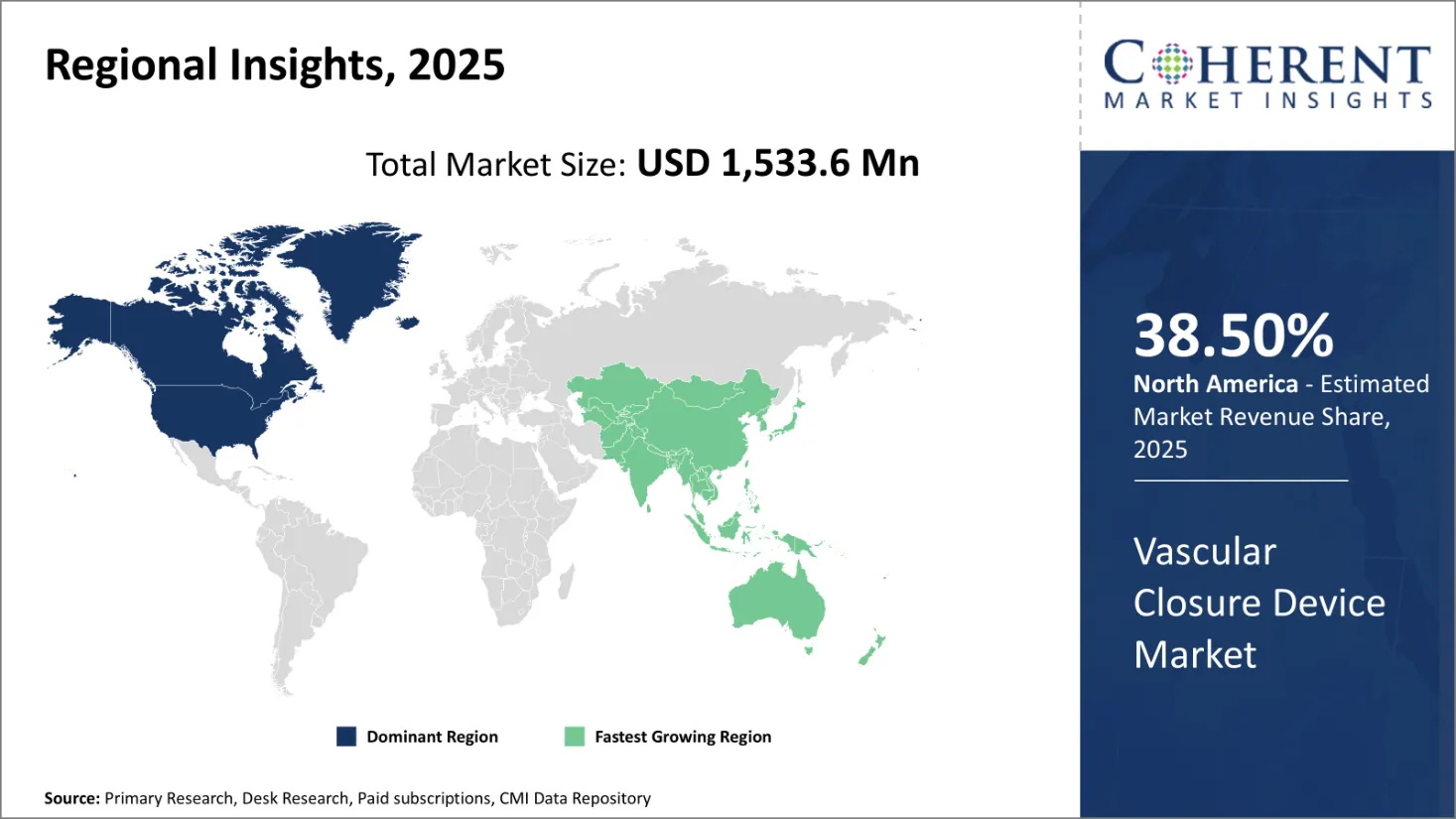
Vascular Closure Device Market size is estimated to be valued at USD 1,533.6 Mn in 2025 and is expected to reach USD 2,321.2 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of6.1% from 2025 to 2032.
The Vascular Closure Devices Market is witnessing significant growth due to several driving factors. A rising number of interventional procedures—such as over 1 million cardiac catheterizations annually in the U.S.—is increasing demand for effective hemostasis solutions. Active closure devices like Perclose ProGlide and Angio-Seal VIP are gaining popularity as they enable early ambulation and same-day discharge, particularly beneficial in outpatient and ambulatory surgical settings.
For instance, a study published in the Journal of Invasive Cardiology found that patients using suture-mediated closure devices ambulated within 2 hours post-procedure compared to 6 hours with manual compression. Hospitals favor these devices for improving patient turnover and comfort.
|
Event |
Description and Impact |
|
Regulatory Approvals and Policy Shifts |
|
|
Clinical Practice Innovations |
|
|
Supply Chain Risks |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Reimbursement plays a key role in the adoption of vascular closure devices (VCDs) globally. Various coding systems support coverage, including ICD-10-PCS codes for vascular occlusion procedures, CPT codes 37191 and 37192 for intravascular filter insertions, HCPCS code G0269 for occlusive device placement, and DRG codes 252–254 classifying vascular procedures by complication severity.
In the U.S., CMS sets reimbursement rates and coverage policies, influencing private insurers. Medicare covers 80% of VCD costs under Part B, with an estimated annual spend of $75 million. Private insurers cover 70-90%, with about $150 million spent annually.
In Europe, the UK’s NICE guides VCD use, with the NHS fully covering approved devices (~£25 million annually). Germany’s Federal Joint Committee (G-BA) governs reimbursement under Statutory Health Insurance, covering 90% of costs with €40 million annual spending.
Japan’s National Health Insurance reimburses 70-90% of VCD costs, spending roughly ¥3 billion annually.
CMS reimburses CPT codes 37191 and 37192 at approximately $2,553 and $1,716 respectively (2021). These reimbursement structures promote VCD use by ensuring cost coverage across major healthcare systems.
Based on product type, the passive vascular closure devices segment is expected to dominate the global vascular closure device market with a 50.0% market share in 2025. This dominance is attributed to their ease of use, compatibility with standard femoral artery access procedures, and low procedural complexity.
Passive devices—including manual compression aids, bandages, and sandbags—enable natural clot formation without requiring active deployment steps, making them a preferred choice across high-volume healthcare settings.
Unlike active vascular closure devices, which require insertion mechanisms or sutures, passive devices are more cost-effective and suitable for settings where procedural simplicity and patient throughput are priorities. Furthermore, they eliminate the risks associated with device misdeployment or infection linked to mechanical closure systems. Their popularity in European markets, where femoral access remains widely used, reinforces their global uptake.
To learn more about this report, Download Free Sample
Leading manufacturers such as Abbott, Terumo, and Cardinal Health continue to invest in R&D and introduce innovative devices tailored to physician preferences. These efforts, along with favorable reimbursement structures and growing outpatient surgical trends, are strengthening market demand. However, challenges such as patent expirations and pricing pressure from insurance payers may impact long-term profitability.
Asia Pacific is emerging as the second-largest and fastest-growing regional market for vascular closure devices, propelled by rising healthcare expenditures, expanding access to interventional procedures, and favorable government initiatives. China holds a dominant position in the region due to its large patient base and modernization of healthcare infrastructure under national policies.
India is also witnessing strong growth, driven by increasing medical tourism, cost-effective manufacturing, and rising procedural volumes. Global medtech leaders are expanding their footprint in these markets, offering localized products. Despite regulatory hurdles and workforce shortages, the region offers significant long-term growth potential.
Europe’s vascular closure device market is led by the passive closure device segment, particularly in countries where femoral artery access remains standard practice. Passive devices—such as compression systems—are favored for their simplicity, cost-effectiveness, and compatibility with standard femoral access techniques.
As procedural safety and ease remain a priority across European healthcare systems, passive VCDs continue to dominate usage. Countries like Germany, France, and the U.K. are major contributors, supported by established healthcare systems and physician familiarity with passive closure techniques.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1,533.6 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.1% | 2032 Value Projection: | USD 2,321.2 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Abbott, Cardiva Medical Inc., Terumo Corporation, Johnson & Johnson Services Inc., B. Braun SE, Biotronik SE & Co. KG, MicroPort Scientific Corporation, ConforMIS, Inc., Medtronic, Transluminal Technologies LLC, Cardinal Health, Teleflex Incorporated, Vasorum Ltd., Tricol Biomedical, Merit Medical Systems, Inc., Stryker |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Greater healthcare spending often correlates with better access to quality care, and this is particularly relevant for the treatment of cardiovascular diseases, which remain a leading cause of death globally. Investment in healthcare can lead to the acquisition of advanced medical devices, training of healthcare professionals, and improved patient care pathways.
In countries with rising healthcare budgets, both public and private hospitals may be more inclined to invest in VCDs, which can streamline the management of vascular access sites after procedures like angiography or percutaneous coronary intervention (PCI). The use of VCDs helps to reduce time to hemostasis, patient recovery time, and overall treatment costs by shortening hospital stays and reducing the need for additional medical intervention due to complications.
Moreover, as out-of-pocket healthcare expenditures decrease due to better funding of health services, patients may be more likely to undergo necessary medical procedures, which would further drive the demand for VCDs. This is especially important in emerging markets, where an increase in disposable income and healthcare funding is making previously unaffordable treatments more accessible.
From a market perspective, companies could leverage increasing healthcare investments by aligning with government initiatives that aim to improve cardiovascular care, forming partnerships with healthcare providers, and working on cost-effective solutions that do not compromise on quality and safety. With this knowledge, companies can better strategize capitalizing on increasing healthcare expenditures to drive the market.
Additionally, new VCDs may cater to a broader range of procedures, including support for complex interventions involving large-bore sheaths or catering to patients with different anatomical challenges. When a new product is introduced, it not only enhances the treatment options available to clinicians but also provides an opportunity for medical facilities to improve their standard of care. This can lead to better patient outcomes and potentially lower the overall cost of cardiovascular care by reducing procedure time, hospital stay duration, and incidence of post-procedure complications.
Moreover, with each innovative product, manufacturers can expand their market presence and establish a reputation as a leader in the market. This can be particularly advantageous when establishing relationships with emerging markets, where healthcare infrastructure is improving, and the demand for quality medical devices is increasing. Constant innovation and refreshment of product lines also help in satisfying the regulatory requirements that may change over time, ensuring compliance with the latest safety standards.
In an industry where regulations are stringent and patient safety is paramount, staying updated with the latest trends and technology is crucial. From a strategic standpoint, companies in the market should invest in robust research and development (R&D) to facilitate the continuous introduction of differentiated products.
The utilization of bioabsorbable vascular closure devices is emerging as a significant trend and represents a promising opportunity within the market. These devices, which are designed to be absorbed by the body over time, are gaining attention for their potential to reduce the risk of infection, minimize inflammation, and improve patient comfort compared to non-absorbable counterparts.
Bioabsorbable VCDs are made from materials that the body can naturally break down and resorb, eliminating the need for a secondary procedure to remove the device. This characteristic is particularly beneficial for patients as it simplifies the post-procedural care and may lead to better long-term outcomes. Moreover, since there is nothing left behind in the body, the chances of chronic foreign body reactions are significantly reduced.
The development and utilization of these bioabsorbable devices are in line with the broader movement towards more biocompatible medical implants and represent the intersection of material science innovation with clinical application. As stakeholders in healthcare continue to prioritize patient safety and quality of care, bioabsorbable VCDs align well with these goals, helping to advance the standard of treatment in interventional cardiology.
These companies will likely benefit from the trend towards more biocompatible and patient-friendly medical devices. For market expansion, it's imperative to not only innovate but also to educate healthcare providers about the benefits and proper usage of these advanced devices. Moreover, regulatory clearances and favorable reimbursement scenarios will play pivotal roles in the adoption of bioabsorbable VCDs across various healthcare systems.
In summary, the emergence of bioabsorbable vascular closure devices is a key trend that stands to significantly impact the market by addressing patient comfort, reducing complications, and supporting the broader shift towards more minimally invasive and patient-friendly medical treatments.
The adoption of hybrid closure procedures is indeed an emerging trend that can drive the global vascular closure device market. Hybrid closure procedures involve the use of multiple techniques or devices to achieve efficient and secure closure of the arterial puncture site after cardiovascular This can include a combination of passive and active closure approaches or integrating different types of technologies to enhance the procedure's success rate and patient outcomes.
Hybrid procedures strive to combine the benefits of various closure methods to optimize hemostasis while minimizing the risk of vascular complications, such as bleeding or arterial occlusion. They are particularly significant for complex cases where a single-method approach may not be sufficiently effective or safe. For instance, a surgeon might use a VCD in conjunction with manual pressure or a topical hemostasis pad to ensure a blood-free and secure closure.
This trend is driven by a continuous effort to improve patient care and reduce the cost burden of post-operative complications. As the healthcare industry progresses, there is a greater appreciation for personalized medicine, which involves tailoring medical treatments to the individual characteristics of each patient. Hybrid closure procedures can be seen as aligning with this philosophy, offering versatility to address varying patient anatomies and conditions.
Manufacturers are recognizing this trend and are likely to invest in product innovations that can cater to hybrid procedural approaches. By providing a suite of complementary products, companies can position themselves as complete solution providers rather than as vendors of a single device type. The rise of hybrid closure procedures highlights the need for ongoing research and training for clinicians to understand the nuances of various VCDs and their synergistic applications.
Moreover, with this trend gaining traction, the market has the potential for increased demand, encouraging further investment in the development and improvement of associated VCD technologies. Keeping an eye on this trend will be essential for market research, as the adoption of hybrid closure procedures represents both a significant opportunity for growth and a shift towards more comprehensive, patient-specific cardiovascular care.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients